In a new study published in Oncogene (2025), researchers investigate the potential of allogeneic invariant natural killer T (iNKT) cells as salvage therapy for a heavily pre-treated metastatic germ cell tumor. The study reports a remarkable case of complete remission in a patient who had exhausted all conventional treatment options, demonstrating the significant promise of iNKT cell therapy in advanced solid tumors. This approach could revolutionize the treatment of refractory cancers, especially for patients with limited alternatives.
This landmark case study reports the first documented complete remission in a heavily pre-treated metastatic germ cell tumor using allogeneic invariant natural killer T (iNKT) cell therapy. The research demonstrates unprecedented clinical success with a novel immunotherapeutic approach in a 49-year-old male patient who had exhausted all standard treatment options.
Title: Salvage therapy with allogeneic invariant natural killer cells in a heavily pre-treated germ cell tumor
Authors: Benjamin Garmezy, Joseph E. Grossman, Jennifer Buell, Thiago Favano, Justin Stebbing, Steven O’Day, Marco A. Purbhoo, Dhan Chand & F. Anthony Greco
Published in Oncogene, 2025
Background: The Science Behind iNKT Cell Therapy
Invariant Natural Killer T (iNKT) cells represent a unique subset of immune cells that bridge innate and adaptive immunity. Despite comprising only 0.01-0.1% of peripheral blood cells, these specialized immune effectors possess remarkable therapeutic potential due to their ability to recognize glycolipid antigens presented by the non-classical MHC molecule CD1d.
The therapeutic agent used in this study, agenT-797, has been developed as an off-the-shelf allogeneic cell therapy with several key advantages:
- No HLA matching required
- No prior lymphodepletion needed
- Excellent safety profile with no reported graft-versus-host disease or cytokine release syndrome
The therapy is currently being evaluated across multiple clinical trials including NCT05108623 (Phase 1 solid tumors), NCT04754100, and NCT06251973 (ongoing Phase 2), with previous successful application in COVID-19 patients (NCT04582201).
Study Design and Methods
The case study focuses on a single patient treated under protocol NCT05108623, which examines the safety and therapeutic efficacy of agenT-797 in patients with relapsed/refractory advanced solid tumors. The study design includes:
- Dose escalation monotherapy cohort
- Combination cohort with immune checkpoint inhibitors
- Administration without prior conditioning or HLA matching
The patient received a single infusion of ex vivo expanded allogeneic iNKT cells (agenT-797) combined with nivolumab as part of the T-787/872 study protocol. Comprehensive monitoring included serum biomarkers, cytokine profiles, and cell persistence tracking using duplex sequencing of donor-specific microhaplotypes.
Patient Case
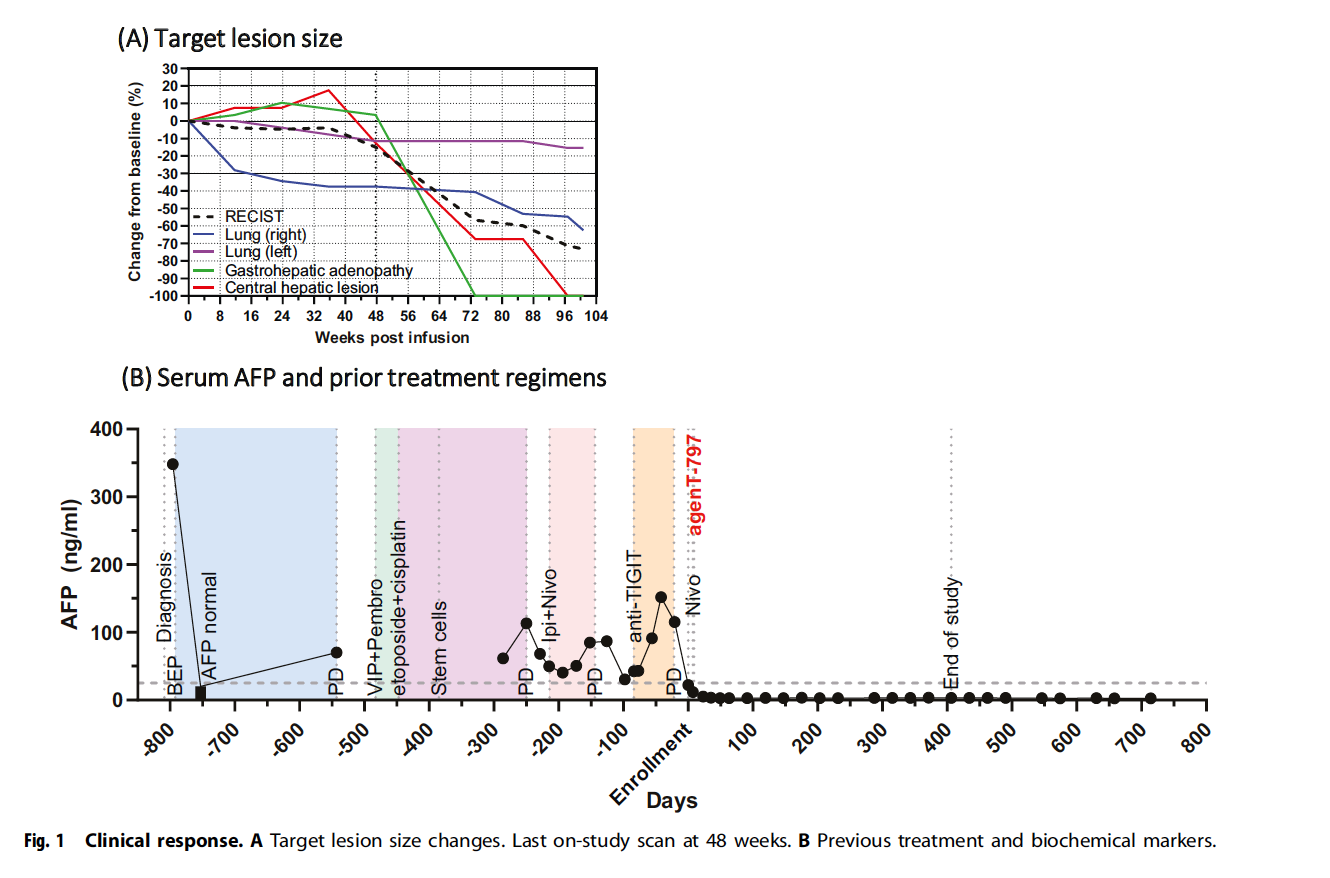
The 49-year-old male patient presented with classic symptoms of advanced malignancy including night sweats, weight loss, cough, right upper quadrant pain, and jaundice. CT imaging revealed multiple liver lesions with significantly elevated tumor markers: lactate dehydrogenase, alpha-fetoprotein (AFP), and beta-human chorionic gonadotropin (β-HCG). Liver biopsy confirmed poorly differentiated non-seminomatous germ cell tumor (testicular cancer metastasis).
The patient underwent an extensive treatment journey spanning over two years:
- First-line therapy with bleomycin, etoposide, and cisplatin (BEP) initially achieved rapid symptomatic improvement and tumor marker normalization. However, disease progression occurred by May 2021 with rising AFP and new lung lesions.
- Second-line therapy consisted of etoposide, ifosfamide, cisplatin (VIP) combined with pembrolizumab, resulting in partial response after three cycles. Pembrolizumab was discontinued after one dose due to persistent tachycardia.
- High-dose chemotherapy with autologous stem cell transplantation was performed in September and November 2021 using etoposide and carboplatin. This achieved only brief partial response and was complicated by severe colitis and sepsis requiring mechanical ventilation.
Additional interventions included stereotactic radiotherapy for brain metastases and subsequent treatment with ipilimumab/nivolumab combined with oral etoposide for four courses, during which tumors progressed. The patient was initially ineligible for an anti-TIGIT trial due to thrombocytopenia, later enrolled but discontinued in November 2022 due to progressive disease.
Key Results with iNKT
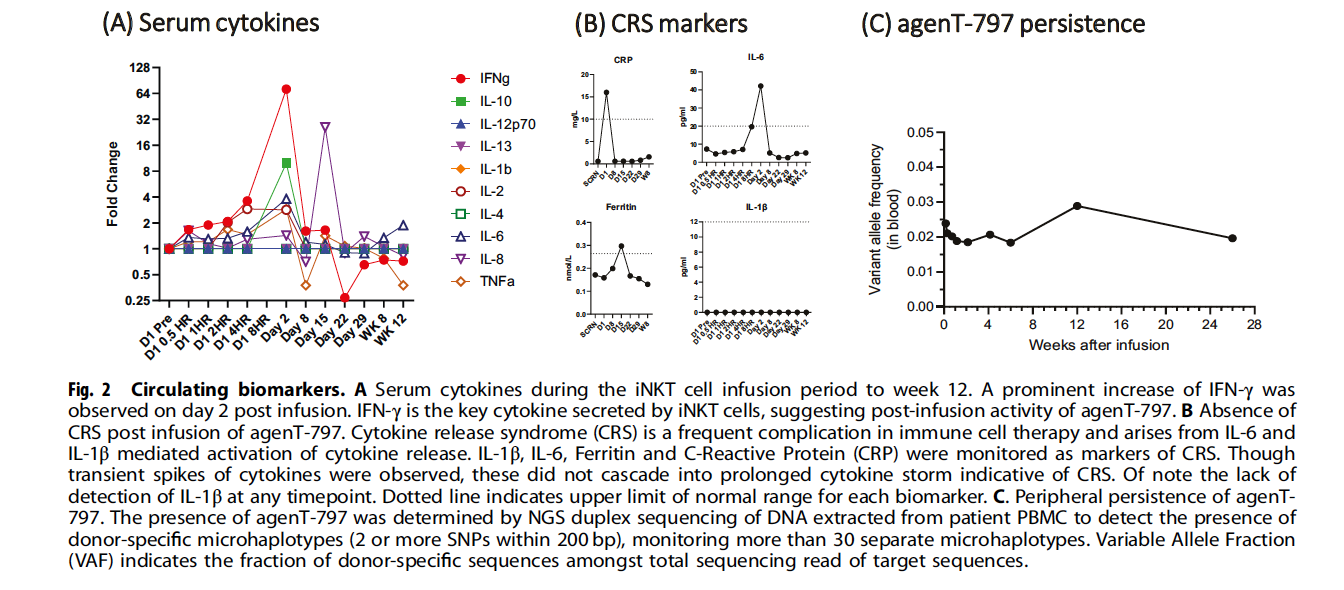
The infusion of allogeneic iNKT cells led to a remarkable clinical response. The patient achieved complete remission both clinically and radiologically, with prolonged normalization of AFP, a key tumor marker. No severe cytokine release syndrome (CRS) was observed, and while there were transient spikes in IL-6, IL-1β, and ferritin levels, these did not result in prolonged cytokine storms. This suggests a favorable safety profile for iNKT cell therapy in this patient population.
The most striking finding was the dramatic tumor reduction across all measured lesions:
- Right lung lesion: >90% reduction
- Left lung lesion: >80% reduction
- Gastrohepatic adenopathy: >70% reduction
- Central hepatic lesion: >60% reduction
Complete remission was maintained through 48-week follow-up, representing the longest documented response in this heavily pre-treated patient population. Alpha-fetoprotein (AFP) levels, which had been persistently elevated throughout previous treatments, achieved sustained normalization following iNKT cell therapy. The biochemical response directly correlated with radiological improvements and was maintained at the latest follow-up.
Biomarker Analysis and Safety Profile
Cytokine monitoring revealed a characteristic pattern consistent with iNKT cell activation. The most significant finding was a prominent increase in IFN-γ on day 2 post-infusion, which represents the key cytokine secreted by iNKT cells and suggests post-infusion therapeutic activity. Other cytokines including IL-10 and IL-12p70 showed transient elevations without cascading into prolonged cytokine storm.
Safety assessment was particularly notable for the absence of cytokine release syndrome (CRS), a frequent complication in immune cell therapy:
- IL-1β: Undetectable at all timepoints
- IL-6: Minimal elevation (peak <50 pg/ml)
- Ferritin: Remained stable within normal limits (<0.4 nmol/L)
- C-Reactive Protein: Mild elevation (peak <20 mg/L)
Cell persistence was confirmed through 6 months using duplex sequencing of donor-specific microhaplotypes, with variable allele frequency peaking at 0.05% at 4 weeks and sustained at 0.01% through 24 weeks.
Mechanisms of Action: Understanding iNKT Cell Anti-Tumor Activity
The research authors propose multiple mechanisms underlying the therapeutic success. iNKT cells can directly kill CD1d-expressing tumor cells through perforin/granzyme-mediated cytolysis and FasL-dependent apoptosis. Additionally, they produce critical cytokines including IFN-γ that activate other immune effectors such as NK cells and CD8+ T cells, enhancing their cytotoxic functions.
Beyond direct cytotoxicity, iNKT cells play a pivotal role in reshaping the tumor microenvironment to favor anti-tumor immunity. By engaging with CD1d-expressing tumor-associated macrophages and myeloid-derived suppressor cells, iNKT cells reduce the suppressive influence of these populations, enabling better infiltration and function of cytotoxic immune cells.
Clinical Implications and Future Research Directions
This case establishes several important precedents for cancer immunotherapy:
- iNKT cell therapy shows promise for complete remission in heavily pre-treated solid tumors.
- The therapy demonstrates an excellent safety profile, with no graft-versus-host disease or cytokine release syndrome.
- Clinical trials should explore iNKT cell therapy in various solid tumor types.
- iNKT cells’ off-the-shelf availability and universal donor compatibility could simplify treatment access.
- Next-generation approaches, including engineering iNKT cells with tumor-specific receptors, could further enhance therapeutic outcomes.
Study Limitations and Considerations
The authors acknowledge several limitations inherent to case report methodology. As a single patient case, generalizability remains limited, and the concurrent use of nivolumab presents potential confounding effects. The relatively short follow-up period, while impressive at 48 weeks, requires longer-term monitoring to establish durability. Additionally, the need for biomarker identification to predict patient response and the requirement for CD1d expression on tumor cells represent areas for future investigation.
Conclusion
This research article represents a watershed moment in cancer immunotherapy, providing the first documented evidence that iNKT cell therapy can achieve complete remission in metastatic germ cell tumors. The 49-year-old patient’s dramatic transformation from progressive disease through multiple standard therapies to sustained complete remission illustrates the transformative potential of this innovative approach.
The authors conclude that this first-of-its-kind case supports the initiation of clinical trials evaluating allogeneic iNKT cell therapy in solid tumors, even in patients who have exhausted standard treatments. The combination of potent anti-tumor activity, excellent safety profile, and off-the-shelf availability positions this approach as a potentially transformative addition to the oncology treatment arsenal.
Key study findings:
- First reported complete remission in metastatic testicular cancer using iNKT cell therapy
- Sustained response through 48-week follow-up with ongoing biochemical and radiological remission
- Excellent safety profile without cytokine release syndrome or graft-versus-host disease
- Confirmed 6-month cell persistence with characteristic cytokine signature
- Provides compelling evidence for expanded clinical investigation across solid tumor types
This groundbreaking research establishes iNKT cell therapy as a promising new frontier in cancer treatment, offering evidence-based hope for patients with refractory malignancies and setting the stage for broader clinical development programs.

Read Full Article in Nature, Oncogene


