Chimeric antigen receptor (CAR) T-cell therapy represents one of the most transformative advances in oncology in the last decade. Unlike traditional chemotherapies or small-molecule inhibitors, CAR T-cell therapy leverages the patient’s own immune system to mount a highly specific and potent anti-tumor response. It involves genetically modifying T lymphocytes to express synthetic receptors that can identify and eliminate malignant cells. While initially developed for hematologic malignancies, research is expanding rapidly into solid tumors, autoimmune diseases, and beyond. This therapy’s success has redefined the landscape of personalized medicine, offering hope to patients with relapsed or refractory cancers previously considered incurable (June et al., 2018).
Mechanism of Action
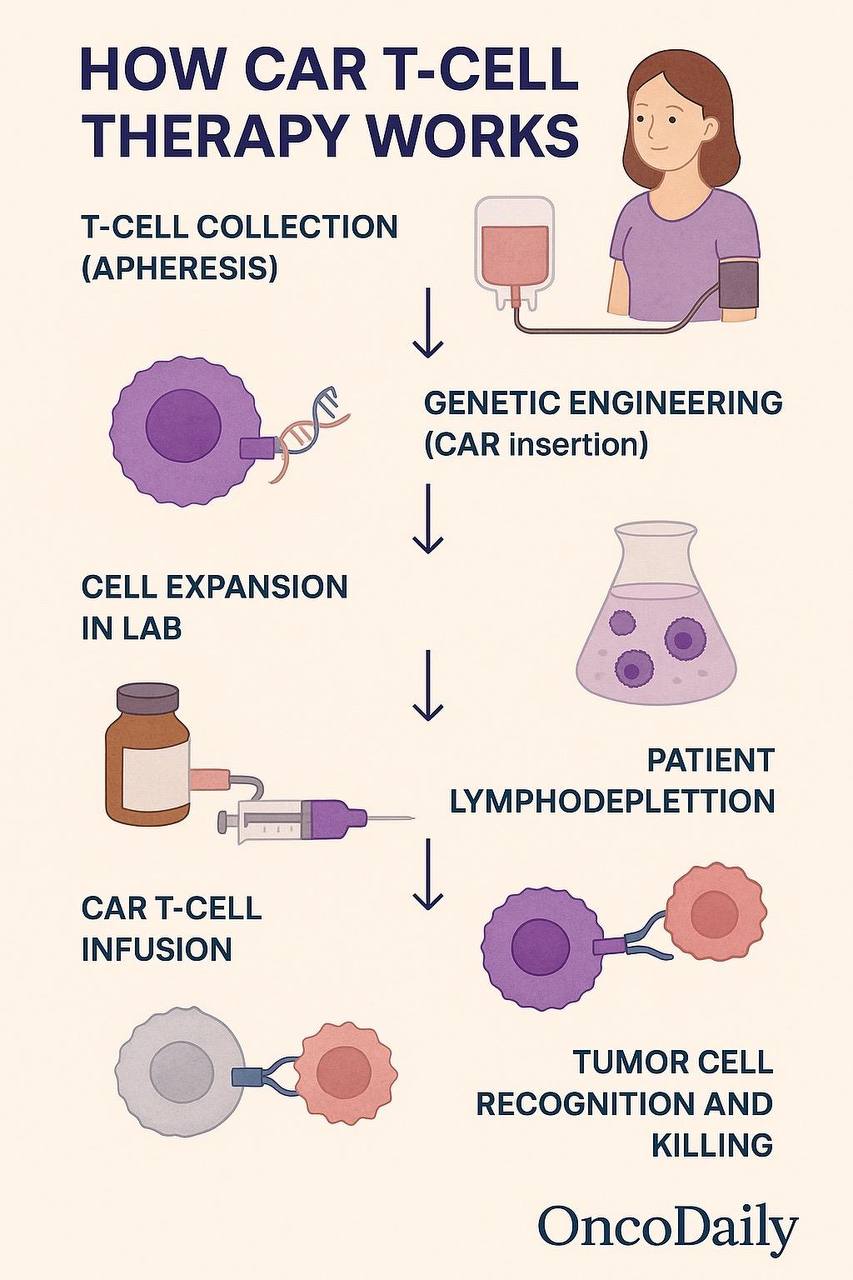
CAR T-cell therapy operates through a sophisticated mechanism that combines genetic engineering with targeted immune activation. At its core, this treatment involves modifying a patient’s autologous T lymphocytes to express a synthetic receptor—a chimeric antigen receptor (CAR)—that redirects the T cells to identify and destroy cancer cells expressing a specific surface antigen. This process bypasses the limitations of natural T-cell receptors (TCRs), particularly their dependence on major histocompatibility complex (MHC) presentation, which is often downregulated in tumor cells as an immune evasion strategy (Sadelain, Riviere, & Riddell, 2013).
The CAR is a modular structure composed of four primary components. First, the extracellular domain includes a single-chain variable fragment (scFv) derived from a monoclonal antibody, which confers specificity for a tumor-associated antigen such as CD19 in B-cell malignancies. Second, a hinge and transmembrane domain connect this scFv to intracellular signaling components. Third, the intracellular signaling domain contains CD3ζ from the TCR complex, which initiates T-cell activation upon antigen binding. Finally, costimulatory domains such as CD28 or 4-1BB (depending on the CAR generation) enhance T-cell proliferation, survival, and cytokine release (Lim & June, 2017).
Upon reinfusion into the patient, CAR T cells begin to circulate, recognizing target antigens on cancer cells independently of MHC presentation. Binding of the CAR to its antigen results in rapid T-cell activation, triggering the release of perforin and granzymes, leading to direct tumor cell lysis. In addition to this cytotoxic function, activated CAR T cells secrete pro-inflammatory cytokines such as IFN-γ, IL-2, and TNF-α, which help recruit other immune cells, sustain T-cell activation, and amplify the antitumor immune response (Newick, O’Brien, Moon, & Albelda, 2016).
One of the hallmarks of CAR T-cell therapy is the capacity for in vivo expansion and persistence. After infusion, the modified T cells can proliferate to high numbers, especially in patients with significant tumor burden. This expansion is a critical determinant of clinical response and durability. In some patients, CAR T cells remain detectable for months or even years post-treatment, providing ongoing immunologic surveillance and reducing the likelihood of relapse (Maude et al., 2018).
Importantly, the evolution of CAR constructs through successive generations has improved both efficacy and safety. First-generation CARs included only the CD3ζ domain and showed limited persistence. Second-generation CARs added a single costimulatory domain such as CD28 or 4-1BB, while third-generation CARs incorporate two costimulatory domains for enhanced signaling. Fourth-generation “armored” CARs are engineered to secrete cytokines like IL-12 or express dominant negative receptors, further augmenting their function within the immunosuppressive tumor microenvironment (Fesnak, June, & Levine, 2016).
In summary, the mechanism of action of CAR T-cell therapy reflects a convergence of immunology and genetic engineering, enabling cytotoxic T cells to effectively target and eliminate tumor cells with high specificity and minimal reliance on conventional antigen presentation. This paradigm shift has redefined therapeutic options for patients with otherwise treatment-resistant malignancies.

Read More About CAR T Cell Therapy for Cancer Treatment on Oncodaily
Approved Indications and Clinical Trials
Since the first regulatory approval in 2017, CAR T-cell therapy has become one of the most significant breakthroughs in the treatment of relapsed or refractory hematologic malignancies. The U.S. Food and Drug Administration (FDA) has approved several CAR T-cell products targeting B-cell lineage antigens, particularly CD19 and B-cell maturation antigen (BCMA), with indications expanding rapidly across adult and pediatric settings.
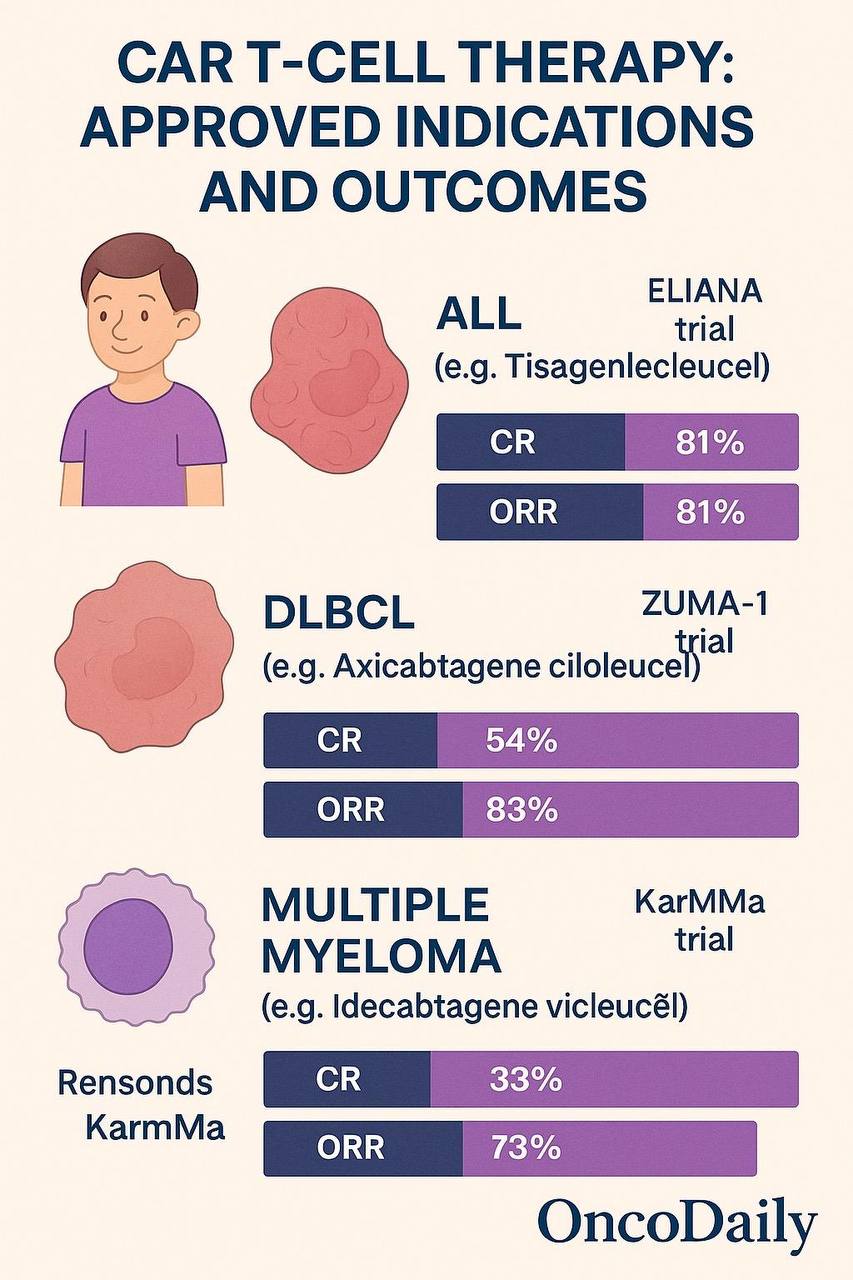
Tisagenlecleucel (Kymriah) became the first FDA-approved CAR T-cell product for pediatric and young adult patients with B-cell acute lymphoblastic leukemia (ALL) that is refractory or in second or later relapse. The approval was based on results from the pivotal ELIANA trial, which demonstrated an overall remission rate of 81%, with a 12-month overall survival of 76% in a population with otherwise dismal prognosis (Maude et al., 2018). This marked a turning point in pediatric oncology, offering long-term remission where prior therapies had failed.
Shortly thereafter, axicabtagene ciloleucel (Yescarta) was approved for relapsed or refractory large B-cell lymphoma based on the ZUMA-1 trial. In this Phase 2 study, 101 patients with refractory diffuse large B-cell lymphoma (DLBCL), primary mediastinal B-cell lymphoma, or transformed follicular lymphoma were treated, achieving an objective response rate of 82% and a complete response rate of 54% (Neelapu et al., 2017). The durability of these responses—nearly 40% of patients remained in remission at 24 months—underscored the curative potential of CAR T-cell therapy in aggressive lymphomas.
Brexucabtagene autoleucel (Tecartus), a CD19-directed CAR T-cell product, received approval for adult patients with relapsed or refractory mantle cell lymphoma (MCL) after at least one prior therapy. In the ZUMA-2 trial, the overall response rate was 93%, with 67% of patients achieving a complete response, a dramatic outcome in a disease where prognosis is typically poor once it becomes refractory (Wang et al., 2020).
For multiple myeloma, idecabtagene vicleucel (Abecma) was the first CAR T-cell therapy targeting BCMA to be approved. This was based on the KarMMa trial, where ide-cel showed an overall response rate of 73%, with 33% achieving a complete response or better in a population with triple-class refractory disease (Munshi et al., 2021). This approval was followed by ciltacabtagene autoleucel (Carvykti), another BCMA-targeting CAR T-cell therapy, which demonstrated an even higher response rate of 97% in the CARTITUDE-1 study, with 67% achieving stringent complete responses and promising durability at 18 months follow-up (Berdeja et al., 2021).
Ongoing clinical trials are further pushing the boundaries of CAR T-cell therapy into earlier lines of treatment and different cancer types. For example, the ZUMA-7 and BELINDA trials compared CAR T-cell therapy to second-line standard of care in patients with relapsed DLBCL. While ZUMA-7 showed significant improvement in event-free survival for axi-cel compared to standard chemotherapy and autologous stem cell transplant (Locke et al., 2022), BELINDA failed to meet its primary endpoint, highlighting the importance of patient selection and therapy timing in clinical success.
Clinical trials are also exploring novel targets such as CD22, CD123, and FLT3, and there is growing interest in allogeneic CAR T cells and multiplexed CAR constructs designed to reduce antigen escape and relapse. Dual-targeting approaches, such as co-expression of CARs against both CD19 and CD22, are being tested to address tumor heterogeneity, especially in B-cell leukemias that lose CD19 expression following treatment (Majzner & Mackall, 2018).
In addition to hematologic malignancies, early-phase clinical trials are underway investigating CAR T-cell therapy in solid tumors such as glioblastoma, pancreatic cancer, and prostate cancer. Although results have been modest thus far due to barriers such as antigen heterogeneity and the immunosuppressive tumor microenvironment, innovations in CAR design and combinatory strategies with checkpoint inhibitors are being pursued to enhance efficacy in these settings.
In summary, CAR T-cell therapy has gained FDA approval for several hematologic malignancies with high response rates and durable remissions in previously untreatable diseases. Ongoing clinical trials are now focusing on refining patient selection, optimizing dosing, and expanding indications, including into solid tumors, which may soon open new avenues for this revolutionary treatment.
Toxicities and Management Strategies
While CAR T-cell therapy has achieved remarkable clinical success, it is also associated with unique and potentially life-threatening toxicities. Understanding and managing these adverse effects is critical to ensuring patient safety and optimizing outcomes. The most common toxicities include cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS), prolonged cytopenias, and infections. These complications arise largely from the mechanism of CAR T-cell activation and expansion within the patient’s immune system.
Cytokine release syndrome (CRS) is the most frequently observed and well-characterized toxicity. It results from massive cytokine secretion following T-cell activation and target cell engagement, primarily involving interleukin-6 (IL-6), interferon-gamma (IFN-γ), and tumor necrosis factor-alpha (TNF-α). CRS typically manifests within days after CAR T-cell infusion and presents as fever, hypotension, hypoxia, and organ dysfunction. Severity is graded according to standardized criteria, such as those proposed by the American Society for Transplantation and Cellular Therapy (ASTCT) (Lee et al., 2019).
The incidence of CRS varies by CAR construct, tumor burden, and disease indication. For instance, in the pivotal JULIET trial evaluating tisagenlecleucel for large B-cell lymphoma, 58% of patients experienced CRS, with 23% experiencing grade ≥3 events (Schuster et al., 2019). The mainstay of treatment for moderate to severe CRS is tocilizumab, an anti-IL-6 receptor monoclonal antibody that can rapidly reverse symptoms. Corticosteroids are used for refractory cases but may impact CAR T-cell efficacy if used excessively or prematurely. Pre-treatment risk assessment—including tumor burden and baseline inflammatory markers—is increasingly utilized to guide monitoring and early intervention strategies.
Immune effector cell-associated neurotoxicity syndrome (ICANS) is another major toxicity, characterized by a spectrum of neurologic symptoms ranging from mild confusion and tremor to seizures, aphasia, and cerebral edema. The exact pathophysiology remains unclear but is believed to involve endothelial activation, blood–brain barrier disruption, and local cytokine effects within the central nervous system. In ZUMA-1, 32% of patients receiving axicabtagene ciloleucel experienced ICANS, with 12% exhibiting grade ≥3 symptoms (Neelapu et al., 2017). Management typically involves high-dose corticosteroids, seizure prophylaxis, and close neurologic monitoring, especially during peak CAR T-cell expansion.
Prolonged cytopenias—particularly neutropenia and thrombocytopenia—are common post-infusion and may persist beyond the initial 30-day period. These cytopenias increase the risk of infections and bleeding complications, necessitating growth factor support, transfusions, and antimicrobial prophylaxis. In the KarMMa trial evaluating idecabtagene vicleucel in multiple myeloma, grade ≥3 neutropenia and thrombocytopenia were observed in 89% and 52% of patients, respectively (Munshi et al., 2021). The causes are multifactorial, involving lymphodepletion, marrow infiltration, and cytokine-mediated suppression.
Infectious complications also pose a significant risk due to the combination of lymphodepleting chemotherapy, CAR T-cell–induced cytopenias, and hypogammaglobulinemia from B-cell aplasia. Prophylactic measures include antiviral and antifungal agents, immunoglobulin replacement, and surveillance cultures. Post-treatment vaccination schedules are increasingly being integrated into survivorship care plans to restore immune competence (Hill et al., 2018).
Other emerging toxicities include tumor lysis syndrome (TLS), macrophage activation syndrome (MAS), and rare autoimmune phenomena. In the context of solid tumors, on-target off-tumor toxicity is a major theoretical concern, where normal tissues expressing the target antigen could become collateral damage. For example, trials targeting HER2 or GD2 have demonstrated pulmonary or neurologic toxicities due to low-level expression of these antigens in normal tissues (Morgan et al., 2010).
Risk mitigation strategies are evolving alongside improved CAR designs and dosing protocols. Fractionated dosing, use of suicide switches (e.g., inducible caspase-9), and engineered “logic-gated” CARs that require dual antigen engagement are under active investigation. Furthermore, guidelines from professional societies such as the ASTCT and the European Society for Blood and Marrow Transplantation (EBMT) provide frameworks for toxicity grading, standardized management algorithms, and hospital readiness protocols.
In summary, although CAR T-cell therapy can induce serious toxicities, the development of early recognition tools, standardized management strategies, and engineering improvements have greatly improved the safety profile of these therapies. A multidisciplinary approach involving oncologists, intensivists, neurologists, and pharmacists is essential for the successful administration and management of CAR T-cell–related complications.
Challenges in Solid Tumors
Despite the transformative success of CAR T-cell therapy in hematologic malignancies, its application in solid tumors remains a formidable challenge. The complexity of the solid tumor microenvironment, antigen heterogeneity, and physical barriers to T-cell infiltration have significantly hindered the therapeutic efficacy of CAR T cells in this setting.
One of the primary obstacles is antigen selection. In contrast to hematologic cancers, where lineage-specific markers such as CD19 or BCMA can be targeted with minimal off-tumor toxicity, solid tumors often lack truly tumor-specific antigens. Most antigens expressed on tumor cells are also found, to varying degrees, on healthy tissues. This creates a high risk of on-target, off-tumor toxicity, where normal organs expressing low levels of the target antigen become unintended victims of CAR T-cell activity (Morgan et al., 2010). For instance, a case of fatal pulmonary toxicity occurred when HER2-targeted CAR T cells were infused into a patient whose normal lung epithelial cells expressed low levels of HER2.
Additionally, antigen heterogeneity within and across tumor lesions can lead to immune escape. Tumor cells may downregulate or entirely lose the target antigen under selective pressure from CAR T-cell attack. This phenomenon has been well documented in CD19-negative relapses in B-cell malignancies and is even more problematic in solid tumors, where genetic and epigenetic variability creates a patchwork of antigenic profiles (Majzner & Mackall, 2018).
Another critical issue is the tumor microenvironment (TME), which in solid tumors is highly immunosuppressive. Components such as tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), regulatory T cells (Tregs), and inhibitory cytokines like TGF-β and IL-10 actively dampen T-cell activity. Furthermore, the expression of immune checkpoints like PD-L1 within the TME can lead to CAR T-cell exhaustion or anergy (Newick et al., 2016). Strategies to overcome these barriers include combination therapies with checkpoint inhibitors or engineering CAR T cells with “armored” features such as PD-1 knockout or secretion of pro-inflammatory cytokines like IL-12.
Physical barriers also impair CAR T-cell trafficking and infiltration into tumor sites. Unlike hematologic cancers where malignant cells circulate freely, solid tumors present structural challenges such as dense extracellular matrix, abnormal vasculature, and hypoxic cores that limit immune cell entry and survival (Salmon et al., 2012). Techniques to overcome these include engineering CAR T cells to express chemokine receptors that match tumor-secreted ligands, such as CCR2 or CXCR1, or delivering them intratumorally to bypass systemic barriers.
The lack of consistent expansion and persistence of CAR T cells in solid tumor patients is another issue. Hematologic cancers often allow for robust in vivo expansion due to abundant antigen presence, but in solid tumors, limited antigen availability and the suppressive TME can prevent effective proliferation and long-term survival of infused T cells. Modifications such as the incorporation of co-stimulatory domains (e.g., 4-1BB or CD28), cytokine signaling modules (e.g., IL-15), and gene edits to enhance metabolic fitness are being explored to address these deficits.
Lastly, clinical trial design and patient selection remain critical. Many early-phase trials in solid tumors have enrolled patients with bulky, refractory disease, where even the most potent immunotherapy might be insufficient. Timing of intervention, tumor burden, and prior treatments likely influence the probability of response, and better stratification tools are needed.
In summary, while solid tumors present formidable challenges to CAR T-cell therapy—including antigen heterogeneity, immunosuppressive environments, physical barriers, and limited persistence—ongoing innovations in CAR design, delivery methods, and combination strategies offer promising avenues. Overcoming these barriers will be essential to extending the benefits of CAR T-cell therapy beyond hematologic malignancies.
Future Directions in CAR T-Cell Therapy
Chimeric antigen receptor (CAR) T-cell therapy has evolved from an experimental concept into a pivotal pillar of modern cancer immunotherapy. While current therapies have revolutionized outcomes in certain hematologic malignancies, there is significant room for growth and refinement. The future of CAR T-cell therapy lies in expanding its applicability across a broader range of malignancies, improving safety and persistence, overcoming resistance mechanisms, and making the therapy more accessible and cost-effective worldwide.
One promising avenue is the development of next-generation CAR constructs. These engineered T cells incorporate sophisticated molecular switches, logic gates, and payloads to improve precision and reduce toxicity. For instance, “armored” CAR T cells can secrete cytokines like IL-12 or express checkpoint blockade molecules to resist immunosuppression in the tumor microenvironment (Chmielewski et al., 2014). Dual-antigen targeting CARs—using either tandem constructs or bispecific logic gates—are being developed to address antigen escape and reduce off-tumor toxicity by requiring co-recognition of two tumor-associated antigens for full activation (Kloss et al., 2013).
Universal or off-the-shelf CAR T cells represent another transformative development. These allogeneic CAR T-cell products are derived from healthy donors and gene-edited to avoid graft-versus-host disease and host rejection. This approach eliminates the need for individualized manufacturing, dramatically shortening time-to-treatment. Trials of allogeneic CD19-directed CAR T cells, such as ALLO-501 and UCART19, have shown early safety and efficacy signals (Benjamin et al., 2020). Genome editing technologies like CRISPR-Cas9 and TALENs play a key role in creating these “universal” T-cell products by knocking out endogenous T-cell receptors and HLA molecules.
Efforts are also underway to expand the role of alternative immune cells, including CAR-natural killer (NK) cells, CAR-macrophages, and invariant natural killer T (iNKT) cells. These cell types may possess inherent advantages in trafficking, safety, and tumor recognition. For example, CAR-NK cells do not induce graft-versus-host disease, have potent innate cytotoxicity, and can be derived from umbilical cord blood or iPSC sources, enabling scalable manufacturing (Liu et al., 2020).
Read About iNKT Cell Therapy on OncoDaily
The integration of synthetic biology is opening new frontiers for cellular programming. Researchers are designing inducible CARs that can be activated or silenced by small molecules, allowing temporal control over T-cell activity. Others are engineering T cells with “suicide switches” to quickly eliminate the cells in case of severe toxicity. Synthetic gene circuits that enable CAR T cells to process environmental cues, make decisions, and carry out complex behaviors are at the cutting edge of immunoengineering (Roybal et al., 2016).
Combining CAR T-cell therapy with other treatment modalities—such as immune checkpoint inhibitors, radiation therapy, and oncolytic viruses—is a key area of investigation. These combinations may overcome the limitations seen in solid tumors by enhancing T-cell infiltration, persistence, and local activation. For instance, checkpoint blockade may reverse T-cell exhaustion, while low-dose radiation can modulate the tumor microenvironment to improve immune recognition (Rafiq et al., 2020).
In parallel, predictive biomarkers and real-time monitoring tools are being developed to identify patients most likely to benefit from CAR T-cell therapy and to track treatment response. Molecular profiling of tumor antigens, cytokine levels, and CAR T-cell kinetics in blood may allow for tailored interventions and earlier recognition of resistance or relapse (Fraietta et al., 2018).
On a broader level, global access and affordability remain urgent challenges. CAR T-cell therapy is complex, costly, and logistically intensive, limiting availability to patients in high-resource settings. Initiatives to decentralize manufacturing, simplify protocols, and reduce cost—such as point-of-care manufacturing platforms and automation—are being pursued to democratize access. The establishment of regulatory frameworks that allow for regional production and innovation is essential to global equity.
In conclusion, the next decade of CAR T-cell therapy promises dramatic evolution. With advances in genetic engineering, synthetic biology, manufacturing, and systems immunology, the therapy is poised to expand its impact across both hematologic and solid tumors. As science continues to refine CAR T-cell design and clinical implementation, the hope is to not only increase response rates but to offer durable cures to a much broader population of cancer patients worldwide.
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD


