
New Paper Alert: Real-World Study Confirms Chemoimmunotherapy Improves Survival in Resectable NSCLC, But Usage Remains Low
Original Study Title: Clinical Outcomes of Perioperative Immunotherapy in Resectable Non–Small Cell Lung Cancer
Authors: Aakash Desai, MD, MPH; Karen Schwed, MSN; Laurynas Kalesinskas, PhD; Qianyu Yuan, PhD; Jonathan Bryan, MS; Catherine Keane, BSN, MSN; Erin Fidyk, MSN, MBA; Emily Castellanos, MD, MPH; Aaron B. Cohen, MD, MSCE; Katherine Harrison, BA; George Ho, BS; Anca Marinescu, BS; Ticiana A. Leal, MD
Published in JAMA Network Open, on June 30, 2025
This article summarizes new evidence on the clinical effectiveness of chemoimmunotherapy in resectable NSCLC, specifically for patients with stage II to IIIA disease. Based on a nationwide real-world cohort, the study compared outcomes of neoadjuvant and adjuvant chemoimmunotherapy, highlighting its impact on distant metastasis–free survival and identifying gaps in implementation. The findings emphasize both the benefit and underutilization of this FDA-approved approach in early-stage lung cancer.
Introduction
Lung cancer remains the leading cause of cancer-related deaths globally, and non–small cell lung cancer (NSCLC) accounts for nearly 85% of these cases. Despite the integration of platinum-based chemotherapy, recurrence remains a significant concern in early-stage resectable NSCLC. In recent years, the addition of immunotherapy to perioperative regimens has shown promise in improving patient outcomes, prompting multiple FDA approvals. However, real-world evidence regarding its adoption and efficacy has been limited until now.
Study Design
This retrospective cohort study analyzed real-world data from the Flatiron Health electronic health record (EHR)–derived database, which aggregates longitudinal oncology data from over 280 cancer centers and approximately 800 care sites across the United States. The goal was to assess the clinical outcomes associated with perioperative chemoimmunotherapy in patients with resectable stage II to IIIA non–small cell lung cancer (NSCLC).
Eligibility Criteria:
- Adults aged ≥18 years with newly diagnosed stage II–IIIA NSCLC
- Underwent curative-intent surgical resection between January 2021 and October 2023
- Received FDA-approved neoadjuvant or adjuvant chemoimmunotherapy after regulatory approval dates
- No evidence of metastatic disease at diagnosis
Treatment Cohorts:
- Neoadjuvant group: Received checkpoint inhibitor (nivolumab or pembrolizumab) with platinum-doublet chemotherapy prior to surgery. Therapy initiation followed FDA approval (April 2022 and October 2023).
- Adjuvant group: Treated with atezolizumab or pembrolizumab (with or without chemotherapy) within 12 weeks after surgery, consistent with 2021–2023 approvals.
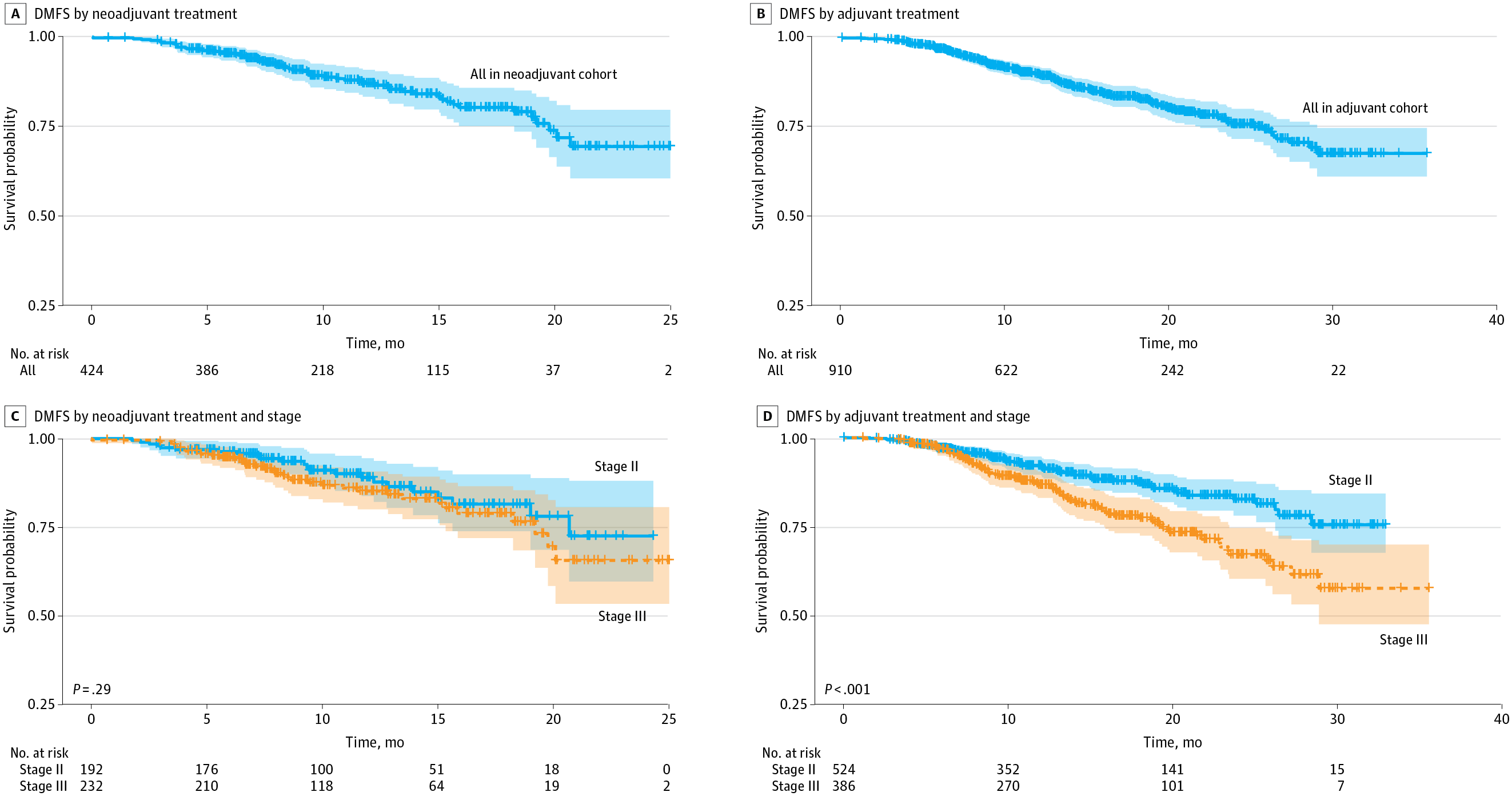
Figure 2 From Original Article. Clinical Distant Metastasis–Free Survival (DMFS) by Neoadjuvant or Adjuvant Treatment and Cancer Stage
The primary endpoint was clinical distant metastasis–free survival (DMFS) at 18 months, defined as time from initiation of systemic therapy to either distant metastasis or death. Secondary endpoints included biomarker testing rates, time to treatment initiation, patterns of metastasis, and the association between duration of immunotherapy and survival outcomes.
Statistical Analyses:
- Kaplan-Meier survival curves for DMFS
- Cox proportional hazards regression adjusted for stage, age, PD-L1 expression, histology, sex, and practice type
Results
The final analytic cohort included 1334 patients with resectable stage II–IIIA NSCLC who received perioperative chemoimmunotherapy:
- Neoadjuvant cohort: 424 patients (31.8%)
- Adjuvant cohort: 910 patients (68.2%)
The median age was approximately 68–69 years across both cohorts, with balanced gender distribution and a high prevalence (>90%) of current or former smokers. Patients in the neoadjuvant cohort had a higher proportion of stage IIIA disease, reflecting more aggressive treatment sequencing.
18-month DMFS:
- Neoadjuvant: 80.2% (95% CI, 75.0–85.7%)
- Adjuvant: 83.0% (95% CI, 80.0–86.0%)
Stage II
- Neoadjuvant: 81.5%
- Adjuvant: 87.3%
Stage IIIA
- Neoadjuvant: 78.9%
- Adjuvant: 77.4%
One of the more striking findings was the impact of immunotherapy duration in the adjuvant cohort. Patients who completed ≥12 months of immunotherapy had a remarkably high DMFS of 95.2%, whereas those treated for <3 months had a DMFS of only 59.2%. This suggests a strong dose-response relationship, underscoring the importance of completing therapy when tolerated.
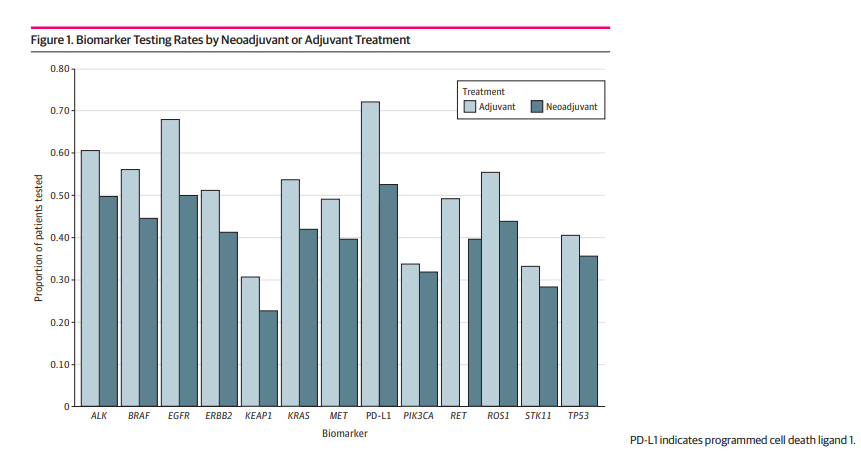
Despite the clear benefits, biomarker testing remained suboptimal, especially in the neoadjuvant group:
- PD-L1 testing: 52.6% (neoadjuvant) vs 72.2% (adjuvant)
- EGFR mutation testing: 50% vs 68%
- ALK rearrangement testing: 49.8% vs 60.7%
Limited biomarker profiling restricts personalized treatment planning, particularly as EGFR/ALK-positive patients derive little benefit from immunotherapy and are better suited for targeted therapies. Patterns of distant metastases were similar in both cohorts. The most frequent sites of recurrence included the brain (34.8%), bone (28%), and pleura (14.4%)—highlighting common routes of failure even after systemic therapy and surgery.
Finally, the study observed a slow yet notable increase in chemoimmunotherapy use over time. From 2022 to 2023, neoadjuvant uptake rose from 8.4% to 13.8%, and adjuvant from 19.7% to 22.6%. However, even in 2023, less than 30% of eligible patients received these FDA-approved regimens—indicating that real-world implementation continues to lag behind clinical guidelines.
Key Takeaways & Conclusion
- Chemoimmunotherapy is effective: Both neoadjuvant and adjuvant approaches led to improved 18-month DMFS, especially in stage II disease for adjuvant and stage IIIA for neoadjuvant therapy.
- Underutilization persists: Despite regulatory approvals, real-world uptake remains low, pointing to barriers in guideline implementation, access, and clinician awareness.
- Biomarker testing is essential: Low testing rates for EGFR, ALK, and PD-L1 hinder appropriate patient selection and may lead to suboptimal outcomes, particularly in populations who derive no benefit from immunotherapy (e.g., EGFR-mutant NSCLC).
- Longer immunotherapy improves outcomes: A strong association between longer treatment duration and better survival in the adjuvant setting suggests the need for full-course adherence.
Clinical recommendations
- Multidisciplinary evaluation is critical for early-stage NSCLC.
- Better integration of biomarker testing into pre-treatment workflows is urgently needed.
- Policies should support testing access and streamline reimbursement.
In summary, this nationwide cohort study offers robust real-world evidence confirming the benefit of chemoimmunotherapy in resectable NSCLC while also shedding light on critical implementation gaps. As perioperative immunotherapy becomes standard care, improving access and adherence will be vital for maximizing patient outcomes.
You can Read The Full Article on JAMA Network Open
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
