TOP Immunotherapy trials from ESMO GI 2025
At the ESMO GI 2025 Congress, three pivotal clinical trials provided new data that could shape the future of immunotherapy across gastrointestinal (GI) cancers. From perioperative treatment in resectable gastric cancer to expanded access in advanced hepatocellular carcinoma (HCC), and a novel approach in immunotherapy-resistant pancreatic cancer, the MATTERHORN, SIERRA, and EXPEL PANC trials each target an unmet clinical need using innovative immune-based strategies.
MATTERHORN Trial: Durvalumab + FLOT in Resectable Gastric/GEJ Adenocarcinoma
Presented by Dr. Salah-Eddin Al-Batran, the Phase 3 MATTERHORN trial (NCT04592913) evaluated the addition of durvalumab, an anti–PD-L1 antibody, to perioperative FLOT chemotherapy (fluorouracil, leucovorin, oxaliplatin, docetaxel) in patients with resectable gastric or gastroesophageal junction (G/GEJ) adenocarcinoma.
-
Event-Free Survival (Primary Endpoint): Durvalumab + FLOT showed a significant improvement in EFS compared to FLOT alone (HR = 0.71; p < 0.001). Median EFS was not reached in the durvalumab arm vs. 32.8 months with placebo.
-
Overall Survival (OS): Favored durvalumab (HR = 0.78; p = 0.025), though not statistically significant at interim analysis.
-
Safety Profile: Comparable Grade 3/4 adverse event rates (~71%) between arms. Immune-related AEs were seen in 25.5% of the durvalumab arm.
-
Quality of Life: No significant differences in patient-reported outcomes between the arms.
Conclusion: These results support durvalumab + FLOT as a potential new perioperative standard of care for resectable G/GEJ adenocarcinoma, improving EFS without compromising quality of life or safety.

You can read the full article here
What People Say?
Mohit Manrao, SVP, Head of US Oncology at AstraZeneca, shared on LinkedIn:
“This post is intended for a US audience:
AstraZeneca announced today that the FDA has granted Priority Review for its supplemental Biologics License Application (sBLA) for the treatment of patients with resectable, early-stage and locally advanced (Stages II, III, IVA) gastric and gastroesophageal junction (GEJ) cancers. Patients in the MATTERHORN Phase III trial who received the regimen saw improvement in event-free survival: a 29% reduction in risk of disease progression, recurrence, or death compared to chemo alone.
Approximately one in four gastric cancer patients experience a recurrence within one year, even with curative intent surgery. But the MATTERHORN study is showing potential to transform event-free survival outcomes.
We’re hopeful that moving treatment earlier and attacking gastric/GEJ cancers from multiple angles will lead to better outcomes for patients. We’re encouraged by the FDA’s Priority Review and look forward to the next steps.
Read more about today’s announcement.”
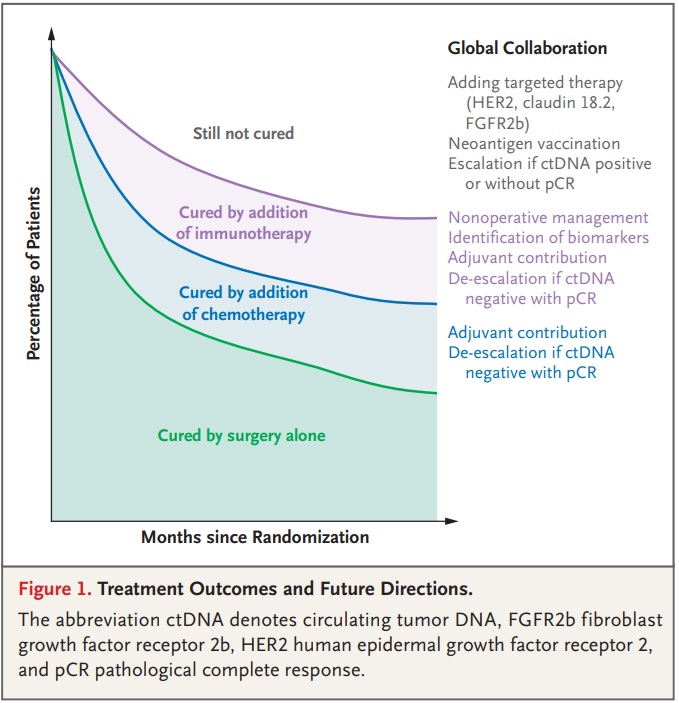
Kohei Shitara, Medical oncologist at National Cancer Center Hospital, shared on X about a paper by Yelena Y. Janjigian et al. published in NEJM:
“It’s my great honor to write the editorial for the pivotal MATTERHORN study NEJM, Yelena Y. Janjigian, ASCO.
More global collaboration is needed to further improve outcomes for our patients with gastric/GEJ cancer.”
Title: Perioperative Durvalumab in Gastric and Gastroesophageal Junction Cancer
Authors: Yelena Y. Janjigian, Salah-Eddin Al-Batran, Zev A. Wainberg, Kei Muro, Daniela Molena, Eric Van Cutsem, Woo Jin Hyung, Lucjan Wyrwicz, Do-Youn Oh, Takeshi Omori, Markus Moehler, Marcelo Garrido, Sulene C.S. Oliveira, Moishe Liberman, Victor Castro Oliden, Elizabeth C. Smyth, Alexander Stein, Mehmet Bilici, Maria Lorena Alvarenga, Vadim Kozlov, Fernando Rivera, Akihito Kawazoe, Olivier Serrano, Eric Heilbron, Alejandra Negro, John F. Kurland, Josep Tabernero.
Amol Akhade, Consultant Medical Oncologist at Suyog Cancer Clinics, shared on X:
“MATTERHORN Trial – ASCO 2025 Spotlight
Can perioperative immunotherapy change outcomes in resectable gastric/GEJ cancer? At GI25, MATTERHORN (Durvalumab + FLOT) showed:
- pCR: 19% vs 7%
- T0: 23% vs 11%
- N0: 52% vs 36%
As Per press release: EFS is statistically significant and clinically meaningful. But KN585 – PCR BENIFIT but no EFS gain
Why MATTERHORN may succeed where KEYNOTE-585 fell short:
- 100% got triplet chemo (vs 20% in KN585)
- 60% completed peri-op FLOT (vs 48 % )
Now your turn- put your brain and give me your best guess for the EFS outcome? HR and Gain in median EFS.”
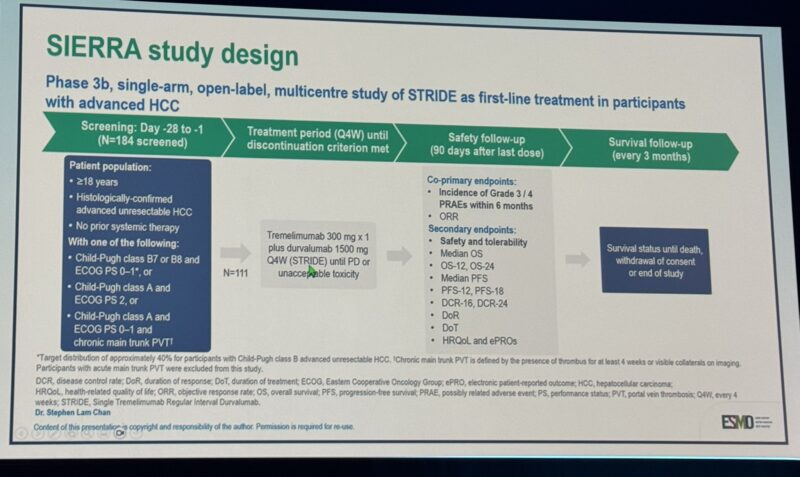
SIERRA Study: Durvalumab + Tremelimumab (STRIDE) in Unresectable HCC with Poor Prognostic Features
The Phase 3b SIERRA trial (NCT05883644), presented by Dr. Stephen L. Chan, evaluated the STRIDE regimen—a combination of durvalumab and a single priming dose of tremelimumab (an anti–CTLA-4 antibody)—in patients with unresectable hepatocellular carcinoma (HCC) and poor prognostic characteristics, including Child-Pugh B7/B8, ECOG PS 2, or main portal vein thrombosis (Vp4).
-
Patient Population: 98 patients, including 35 with CP B7/B8, 44 with ECOG 2, and 19 with Vp4.
-
Adverse Events: Grade 3/4 treatment-related AEs occurred in 19.4% of patients. Serious AEs in 32.7%. Immune-mediated AEs reported in 25.5%.
-
Safety by Subgroup:
-
CP B7/B8: Grade 3/4 PRAEs in 17.1%
-
ECOG PS 2: 18.2%
-
Vp4: 26.3%
-
Conclusion: The STRIDE regimen maintained a manageable safety profile, even in high-risk populations often excluded from trials. These data support STRIDE as a viable immunotherapy approach in broader real-world HCC populations, extending the impact beyond the HIMALAYA study.
You can read the full article here
What People Say?
Lorenza Rimassa, Associate Professor of Medical Oncology at Humanitas University, shared on X:
“Stephen L. Chan presents safety results of the phase 3b SIERRA study of durvalumab and tremelimumab STRIDE in patients with HCC and poor prognosis at ESMOGI25.”
EXPEL PANC: BXCL701 + Pembrolizumab in Second-Line Advanced PDAC
In a disease historically unresponsive to immunotherapy, Dr. Benjamin A. Weinberg presented interim results from the Phase 2 EXPEL PANC trial (NCT05558982) evaluating BXCL701—a first-in-class oral DPP4/8/9 and fibroblast activation protein (FAP) inhibitor—in combination with pembrolizumab, a PD-1 inhibitor, in second-line advanced pancreatic ductal adenocarcinoma (PDAC).
-
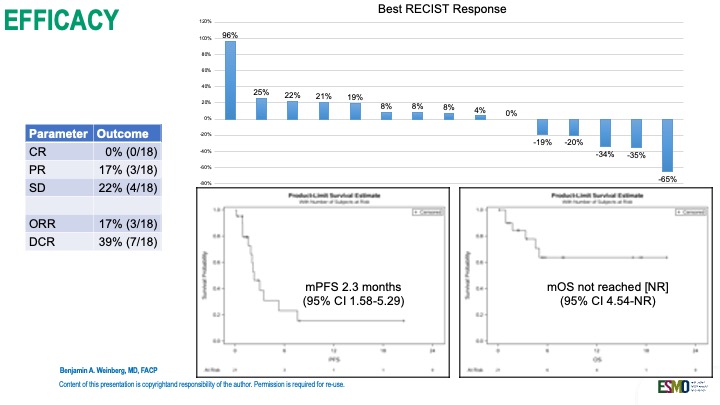
Patient Cohort: 21 patients enrolled; 16 evaluable at 18 weeks.
-
Disease Control Rate (DCR): 39%
-
Partial responses: 17%
-
Stable disease: 22%
-
-
Median PFS: 2.3 months (95% CI: 1.6–5.3)
-
Median OS: Not yet reached
-
Safety: No new or unexpected toxicities observed.
Conclusion: These preliminary findings suggest that BXCL701, by targeting immunosuppressive mechanisms within the tumor microenvironment, may sensitize PDAC to checkpoint blockade. Ongoing biopsy and biomarker analysis may clarify predictors of response in this historically immune-resistant setting.

You can read the full article here
What People Say?
Sharlene Gill, Medical Director, Staff Wellness and Engagement at BC Cancer, shared on X:
“My top picks ESMOGI25 abstracts
- TALENTACE: Atezo+Bev post-TACE in uHCC → mPFS 11 vs 7m, await OS
- EXPEL PANC ph2: BXCL701+pembro 39% DCR in 2L PDAC
- ph2 givastomig CLDN18.2 bispecific with FOLFOX-nivo in mGEC → ORR 71%
- ph2 ipragratinib FGFR4 + atezo: ~50% ORR in FGF19+ HCC
“
Cure GI Cancers shared on X:
“Benjamin Weinberg, presents the EXPEL PANC trial: 2nd line pembro + BXCL701 in metastatic pancreatic cancer: 17% response rate, 39% disease control rate, PFS 2.3 months, OS not reached, 3 patients progression free > 6 months, hope on the horizon for immunotherapy in panc ESMOGI25.”
Benjamin Weinberg presents the EXPEL PANC trial: 2nd line pembro + BXCL701 in metastatic pancreatic cancer.”
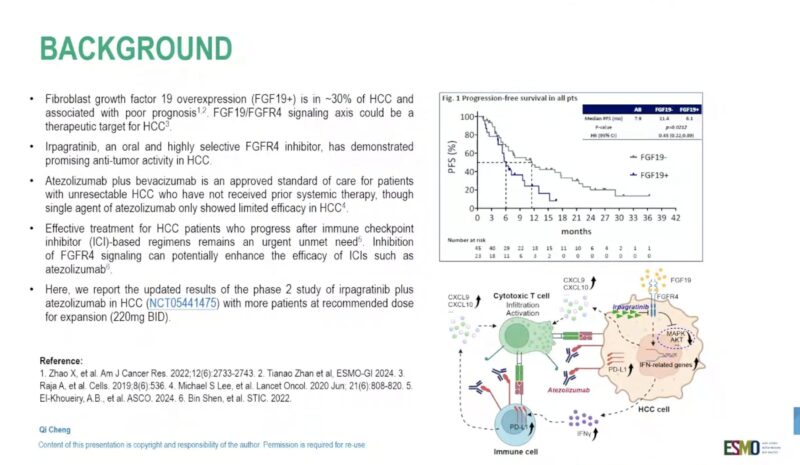
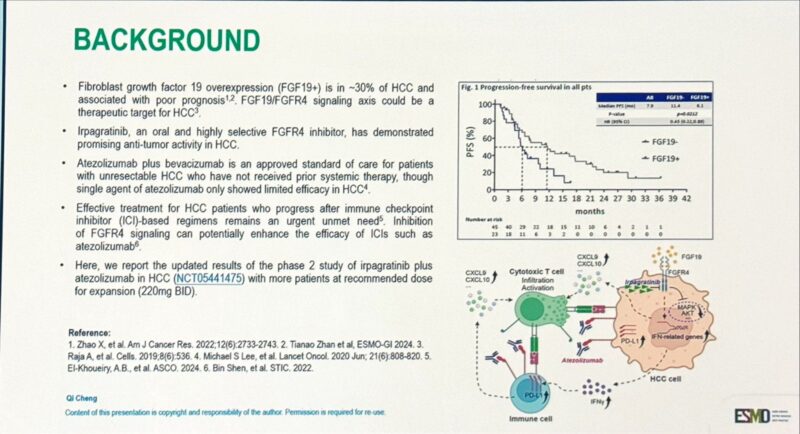
ABSK-011-201 Trial: Irpagratinib + Atezolizumab in Advanced FGF19+ HCC
Presented at ESMO GI 2025 (Abstract 149MO), updated results from the Phase 2 ABSK-011-201 trial evaluated the combination of irpagratinib, an FGFR4 inhibitor, and atezolizumab, a PD-L1 inhibitor, in patients with advanced hepatocellular carcinoma (HCC) and FGF19 overexpression.
- Objective Response Rate (ORR): Among 29 evaluable patients, the confirmed ORR was 51.7%, with responses observed in both first-line (50.0%) and previously treated (52.9%) subgroups. Most responses were ongoing at a median follow-up of 7.1 months, and the median duration of response (DOR) had not yet been reached.
- Progression-Free Survival (PFS): Median PFS was ≥7.0 months, and overall survival data were not yet mature.
- Safety Profile: Treatment-related adverse events (TRAEs) included liver function test elevations, with grade 3 ALT increase in 12.1% and AST increase in 9.1%. There were no grade 4/5 TRAEs and no treatment discontinuations due to toxicity.
- Patient Population: The trial was conducted in China; ~85% of patients were positive for hepatitis B virus (HBV). This highlights the need for further validation in broader global cohorts.
- Expert Commentary: Dr. Rachna Shroff (University of Arizona Cancer Center) noted,
“These are exciting data for patients with FGF19-positive tumours, which are associated with a poor prognosis… We have not reached the median DOR yet. However, additional details about the selection criteria, including how patients were screened for FGF19, would be of interest.”
Conclusion: This early-phase study demonstrates promising activity of irpagratinib + atezolizumab in a molecularly defined subset of HCC with limited therapeutic options. Longer follow-up and biomarker refinement will be key to confirming its potential.
What People Say?
Arndt Vogel, Managing Senior Consultant and a Professor in the Department of Gastroenterology, Hepatology, and Endocrinology at Hannover Medical School, shared on X:
“Irpagratinib (ABSK-011) + atezolizumab in 1L naive and pretreated HCC with FGF19 overexpression
Updated results of phs II ABSK-011-201 study
- ORR 50% vs 52%
- mPFS 7.0 vs 8.3mo
Interesting activity, good safety profile.”
Angela Lamarca, Consultant Medical Oncologist at Jiménez Díaz Foundation University Hospital and The Christie NHS Foundation Trust, shared on X:
“Results from phase II ABSK-011-201 study at ESMOGI25
Irpagratinib (ABSK-011; iFGFR4) + atezolizumab
1L (15pts) and 2L (18pts) FGF19 +ve HCCImpressive ORR >50%
Mental note: ~30% of HCC are FGF19 +ve.”
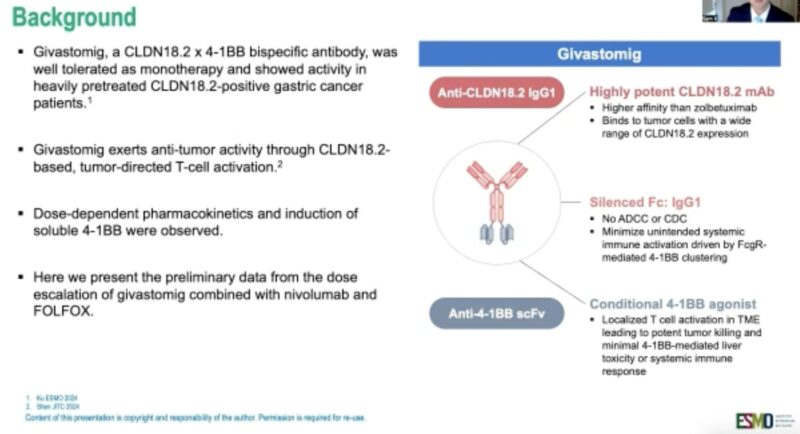
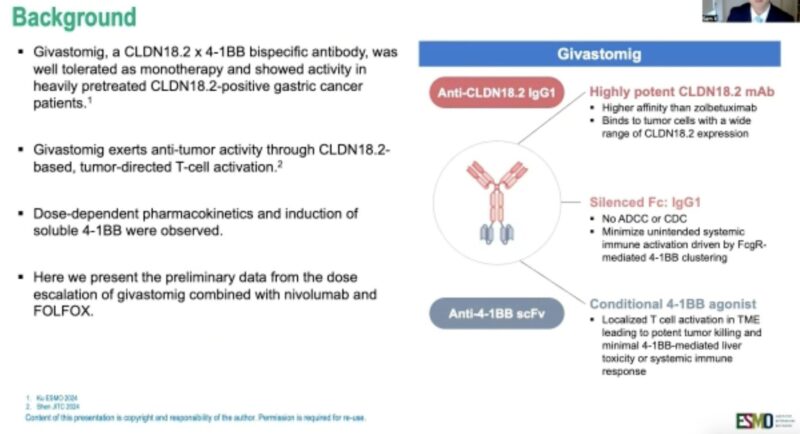
Givastomig + Nivolumab + mFOLFOX6 in CLDN18.2+ Gastric, GEJ, and Esophageal Adenocarcinoma
Presented by Dr. Samuel Klempner at ESMO GI 2025, the phase 1b study (NCT04900818) evaluated givastomig—a bispecific antibody targeting CLDN18.2 and 4-1BB—in combination with nivolumab and mFOLFOX6 in patients with HER2-negative, CLDN18.2-positive advanced gastric, gastroesophageal junction (GEJ), or esophageal adenocarcinoma.
- Objective Response Rate (Primary Efficacy): The combination achieved an ORR of 71% across all evaluable patients (n = 17). In PD-L1 CPS ≥5 patients, the ORR was 82%. High response rates were also observed across CLDN18.2 expression levels (67% for ≥75%; 80% for <75%).
- Progression-Free Survival (PFS): Median PFS and duration of response were not yet reached at 9-month follow-up. Six-month PFS rate was 72.9%.
- Safety Profile: Givastomig was well tolerated at all tested doses (5, 8, 12 mg/kg). Grade ≥3 treatment-emergent adverse events occurred in 65% of patients; 24% were related to givastomig. No dose-limiting toxicities were reported, and no treatment-related deaths occurred.
- Mechanism of Action: Givastomig binds CLDN18.2-positive tumor cells and conditionally activates 4-1BB signaling to stimulate T cells in the tumor microenvironment, while minimizing systemic immune toxicity.
Conclusion: Givastomig plus chemoimmunotherapy demonstrated promising efficacy and manageable safety in CLDN18.2+ upper GI cancers, including in patients with low PD-L1 expression. Expansion cohorts are ongoing.
What People Say?
Arndt Vogel, Managing Senior Consultant and a Professor in the Department of Gastroenterology, Hepatology, and Endocrinology at Hannover Medical School, shared on X:
“Givastomig, a novel claudin 18.2/4-1BB bispecific antibody, in combination with nivolumab and mFOLFOX in mGEC
Phase I/II
ORR 71%
Interesting activity.”
Galip Can Uyar, Medical Doctor at Etlik City Hospital, shared on X:
“Givastomig + Nivolumab + mFOLFOX in mGEC (Phase I/II)
-
Bispecific CLDN18.2/4-1BB antibody
-
ORR: 71% (up to 83% in expansion cohorts)
-
Active in both low PD-L1 and low CLDN18.2
-
Well-tolerated at all doses
-
Deep, durable responses
-
Ongoing 12 mg/kg (NCT04900818)”
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023