Amol Akhade, Consultant Medical Oncologist at Suyog Cancer Clinics, shared a post on LinkedIn:
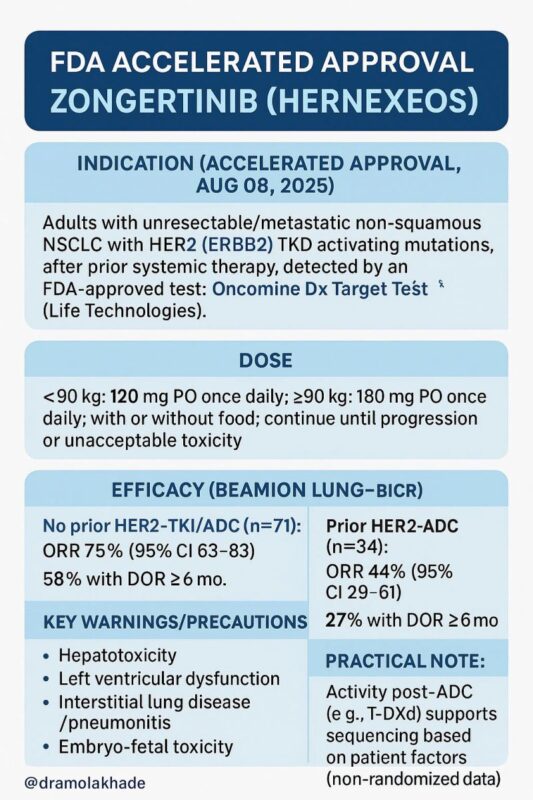
“FDA Accelerated Approval: Zongertinib (Hernexeos)
A new targeted therapy option for a rare molecular subset of lung cancer has just entered our clinic shelves.
On August 8, 2025, the US FDA granted accelerated approval to zongertinib (Hernexeos, Boehringer Ingelheim) for adults with unresectable or metastatic non-squamous non-small cell lung cancer (NSCLC) harboring HER2 (ERBB2) tyrosine kinase domain (TKD) activating mutations, after prior systemic therapy, as detected by an FDA-approved test. The Oncomine Dx Target Test was simultaneously approved as the companion diagnostic.
Why this matters
HER2-mutant NSCLC is uncommon (~2–4% of non-squamous cases) but biologically aggressive, with limited options beyond chemotherapy or antibody–drug conjugates (ADCs). Until now, trastuzumab deruxtecan (T-DXd) was the only FDA-approved HER2-targeted agent in this setting.
Zongertinib now offers:
- Oral administration (once daily) High response rates Activity even after prior HER2-ADC exposure Dosing <90 kg: 120 mg PO once daily ≥90 kg: 180 mg PO once daily With or without food; continue until progression or unacceptable toxicity. Efficacy (Beamion LUNG-1)
- BICR-assessed, RECIST v1.1, in patients with HER2-mutant NSCLC who had progressed after platinum chemo ± immunotherapy:
- No prior HER2-ADC (n=71):
- ORR 75% (95% CI: 63–83) 58% had duration of response ≥6 months
- Prior HER2-ADC (n=34):
- ORR 44% (95% CI: 29–61) 27% had duration of response ≥6 months
Early presentations suggest intracranial activity in patients with brain metastases, an important consideration in NSCLC care.
Key safety points Hepatotoxicity (monitor LFTs regularly) Left ventricular dysfunction (baseline and periodic cardiac evaluation) Interstitial lung disease/pneumonitis (prompt evaluation of new respiratory symptoms) Embryo-fetal toxicity (effective contraception required)
How I see it fitting in
For eligible patients, zongertinib offers a convenient oral option with impressive activity both before and after ADC therapy. In ADC-naïve patients: potential to achieve high ORR without infusion-related logistics.
Post-T-DXd: valuable salvage option with meaningful responses. Choice between T-DXd and zongertinib first may depend on ILD risk, cardiac profile, CNS disease, patient preference, and drug accessibility.
Take-home
The arrival of zongertinib signals the rapid evolution of precision medicine in lung cancer—where HER2 mutation is no longer a biologic orphan. As confirmatory trials read out, we’ll learn whether its benefits extend into earlier lines of therapy and how best to sequence it with ADCs.”
More posts featuring Amol Akhade on OncoDaily.