
MiNK Therapeutics Stock Surges on Breakthrough Testicular Cancer Remission and iNKT Cell Therapy Progress
MiNK Therapeutics is a clinical-stage biotechnology company focused on developing allogeneic invariant natural killer T (iNKT) cell therapies for cancer and inflammatory diseases. Headquartered in New York, MiNK is a subsidiary of Agenus Inc. and is advancing its lead candidate, AgenT‑797, in solid tumors and hematologic malignancies. The company’s approach aims to leverage the unique biology of iNKT cells to deliver off-the-shelf, immune-mediated tumor control with the potential for broad application across multiple cancer types.
The company has experienced dramatic volatility over the past few days.
MiNK Therapeutics Stock Rises on Breakthrough Testicular Cancer Remission Data
MiNK Therapeutics delivered a significant milestone late last week as new clinical data fueled exceptional investor enthusiasm. The company’s stock surged more than 700% on Friday, marking one of the most dramatic daily gains in the biotechnology sector this year.
The rally followed the publication of a case report in the journal Oncogene, detailing a patient with advanced, treatment-refractory testicular cancer who achieved complete remission after receiving MiNK’s lead product, AgenT‑797, in combination with the immune checkpoint inhibitor nivolumab. This unprecedented result underscores the promise of MiNK’s iNKT cell therapy platform, which leverages the unique biology of invariant natural killer T cells to target cancer cells through both direct killing and immune system activation.
You can read our New Paper Alert

About MiNK Therapeutics
MiNK Therapeutics is a biopharmaceutical company focused on the development of innovative immunotherapies for the treatment of cancer and other serious diseases. The company is particularly focused on MiNK Therapeutics, is focused on using the body’s immune system to fight cancer, especially by developing cell-based treatments and therapies that boost the immune system to target cancer cells more effectively.
One of the key platforms of MiNK Therapeutics is the use of natural killer (NK) cells. NK cells are a type of white blood cell that plays a crucial role in the body’s immune response, particularly in identifying and killing tumor cells. MiNK is developing NK cell-based therapies that can be engineered and enhanced to target cancer more effectively.
Dr. Nils Rudqvist, Senior Director of Research, described MiNK’s scientific ambition, noting
“We strive to bring iNKT cells to the next level and beyond, by utilizing our innovative technology and engineering capabilities.”
MiNK Therapeutics has a portfolio of immunotherapy candidates in development, with several in clinical trials. These therapies focus on various types of cancers, including solid tumors and hematologic (blood) cancers. MiNK is committed to advancing its therapies through clinical testing to provide patients with more treatment options, especially those with cancers that are difficult to treat with traditional therapies.
MiNK has gained attention for its innovative treatments, particularly in the area of testicular cancer and immuno-oncology.
“Our allogeneic iNKT platform, agenT-797, has already demonstrated potent, durable activity in solid tumors—without lymphodepletion, genetic modification, or complex conditioning. Building on that, we have shown that our CAR-iNKT cells deliver dual targeting through the invariant TCR and CAR, while actively reshaping the tumor microenvironment. With MiNK-215, our IL-15–armored, FAP-targeting CAR-iNKT therapy, we’re now tackling the stromal barriers that have long prevented immune infiltration in resistant tumors.”
says Jennifer Buell, Ph.D., President and Chief Executive Officer of MiNK Therapeutics, emphasizing the company’s unique approach, stating that MiNK’s pioneering iNKT cell platform.
The company has released promising data from its clinical trials, showing that its treatments can lead to remission in patients who have not responded to conventional treatments.
What is iNKT and the mechanism of action?
Invariant natural killer T (iNKT) cells are a unique, innate‑like subset of T cells that recognize lipid antigens presented by CD1d on antigen-presenting cells or tumor cells. Once activated via their semi-invariant TCR (e.g., Vα24-Jα18/Vβ11 in humans), they rapidly unleash a cytotoxic response that targets malignant or infected cells
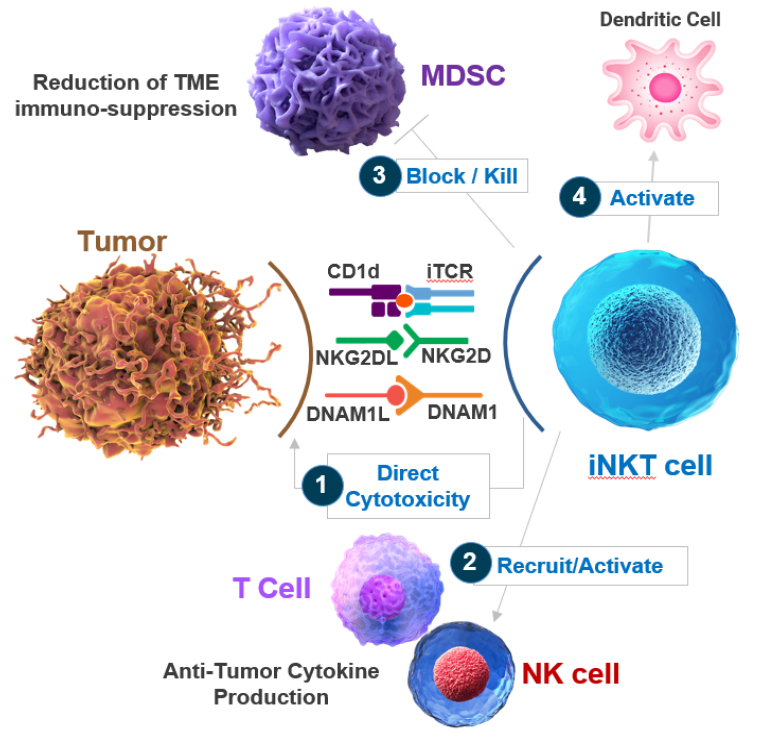
Their cytotoxic toolbox includes:
- Granule exocytosis pathway – they release perforin and granzymes. Perforin forms pores in the target cell membrane or endosomes, allowing granzymes to enter and activate apoptosis-inducing caspase cascades
- Death receptor–mediated killing – they express Fas ligand (FasL) and TRAIL, which bind Fas or TRAIL receptors on target cells to directly trigger apoptosis
- Cytokine-driven amplification – upon activation, iNKT cells secrete large quantities of cytokines like IFN‑γ and GM‑CSF, which enhance dendritic cell activation, recruit NK and CD8⁺ T cells, and broadly remodel the tumor microenvironment
These mechanisms combine immediate, targeted tumor cell killing with powerful immune modulation, making iNKT cells a multifaceted force in tumor immunosurveillance and a compelling platform for cell therapy development
Here you can read our article about iNKT cell therapy
Studies with AgenT‑797
A Study of agenT-797 in Combination With Botensilimab, Balstilimab, Ramucirumab, and Paclitaxel for People With Esophageal, Gastric, or Gastro-esophageal Junction Cancer
At the 2025 ASCO Gastrointestinal Cancers Symposium, MiNK Therapeutics presented TPS515, a Phase II investigator-initiated trial led by Memorial Sloan Kettering, evaluating a novel 5-agent regimen in patients with previously treated, unresectable or metastatic gastroesophageal adenocarcinoma (NCT06251793). The regimen combines AgenT‑797 (allogeneic iNKT cells) with botensilimab (a next-generation CTLA‑4 inhibitor), balstilimab (anti‑PD‑1), ramucirumab, and paclitaxel
Building on encouraging Phase I results in PD‑1–refractory tumors—where patients, including those with gastric cancer, showed meaningful immune activation and clinical responses—this trial aims to assess tolerability and efficacy in a heavily pretreated population. Early signals demonstrate the regimen is well tolerated and shows signs of activity, including robust T‑cell infiltration in responding tumors
GlobeNewswire. A detailed update is expected in the second half of 2025.
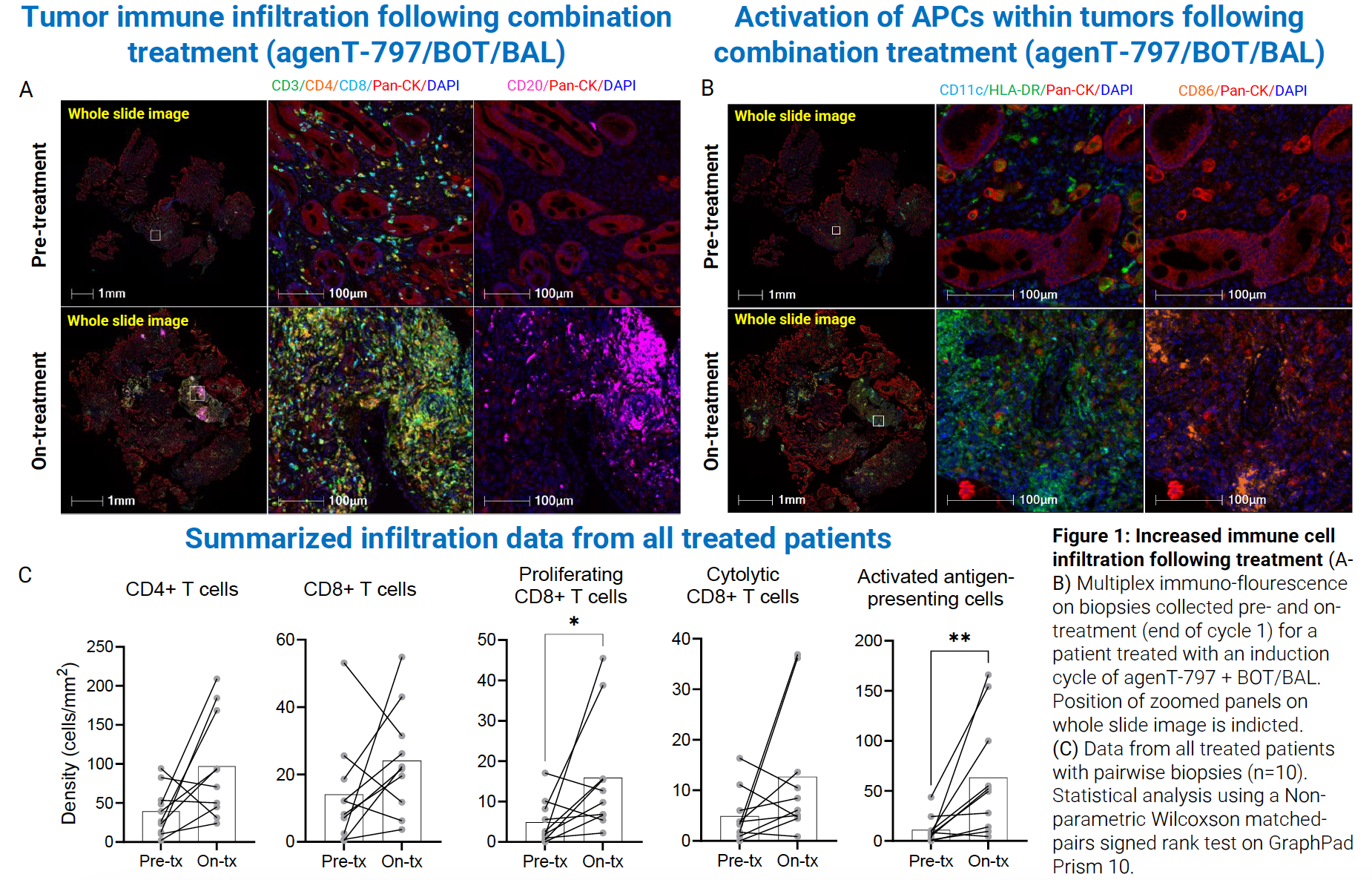
Cytrin et al, 2025, AACR IO
A Study Investigating agenT-797 in Participants With Relapsed/Refractory Solid Tumors
An Oncogene publication (Hadfield et al., March 2024) reports a compelling case of a patient with microsatellite instability‑high (MSI‑H) gastric adenocarcinoma who had progressed on both chemotherapy and anti‑PD‑1 therapy. Following treatment with AgenT‑797—an off‑the‑shelf, allogeneic invariant natural killer T (iNKT) cell therapy—the patient achieved a durable tumor response.
The study highlights how AgenT‑797 harnesses the unique biology of iNKT cells, which recognize tumor-associated lipid antigens via CD1d. Upon infusion, these cells launch multifaceted antitumor activity: directly killing cancer cells through cytotoxic mechanisms like perforin/granzyme and death receptor pathways, while also secreting key cytokines (e.g., IFN‑γ) that recruit and activate other immune effectors such as T cells, NK cells, and dendritic cells.
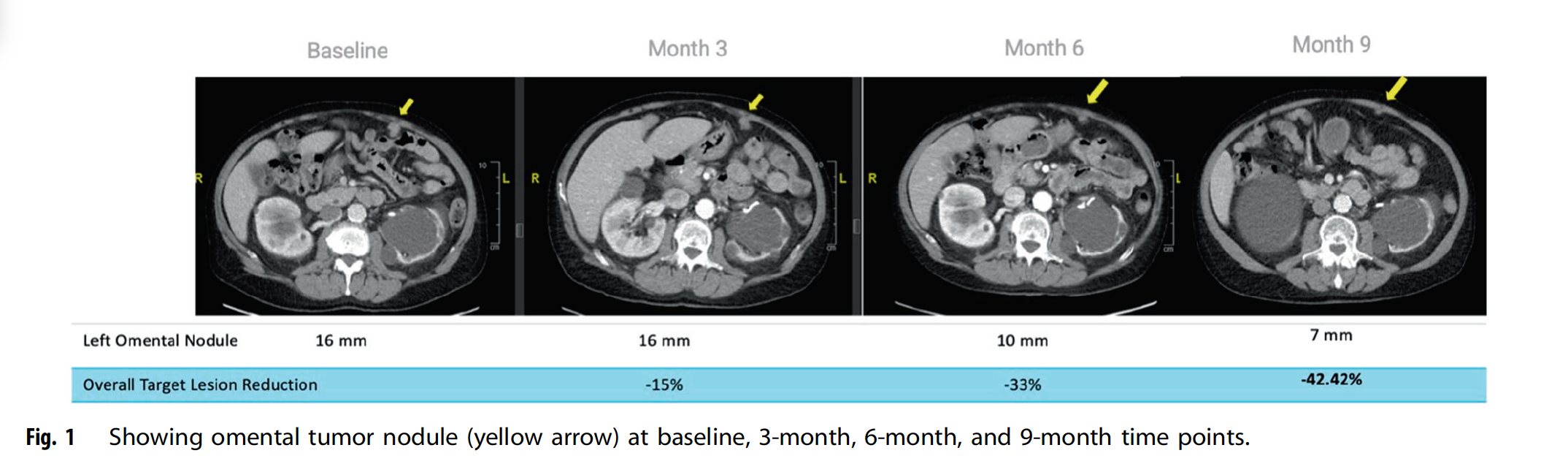
Overall, this case provides proof-of-concept that allogeneic iNKT cell therapy can overcome resistance to PD‑1 checkpoint inhibitors, combining direct cytotoxic action with broad immune potentiation in a heavily pre-treated gastric cancer patient.
A New Era for Cell-Based Cancer Therapies
The sharp rise in MiNK’s share price on Friday reflects strong investor confidence in the transformative potential of iNKT cell therapies and the company’s ability to pioneer a new frontier in immuno-oncology. Investors responded enthusiastically to the promising clinical data, recognizing that AgenT‑797 could represent a major breakthrough for patients with limited treatment options, such as those with advanced, refractory testicular cancer.
Beyond this initial success, MiNK Therapeutics is actively expanding its pipeline, with ongoing studies in a variety of solid tumors, including gastric cancer, where current therapies often fail to provide durable responses. The positive momentum observed last week not only underscores growing excitement around the company’s innovative approach but also signals broader optimism about the potential of allogeneic, off-the-shelf cell therapies to deliver scalable and accessible treatment solutions. As MiNK advances toward future clinical milestones, the company is well positioned to attract continued investor interest, strategic partnerships, and potentially reshape the treatment landscape for difficult-to-treat cancers.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
