
Highlights From ESMO Gastrointestinal Cancers Congress 2025 by Arndt Vogel
The ESMO Gastrointestinal Cancers Congress 2025 is taking place from July 2-5 in Barcelona, Spain, with an online option available through a dedicated virtual platform. Organized by the European Society for Medical Oncology, this key event gathers leading experts, researchers, and healthcare professionals who are focused on advancing treatment and care for gastrointestinal cancers.
Attendees are experiencing groundbreaking presentations, multidisciplinary tumor boards, and lively discussions on the latest research, innovative therapies, and emerging technologies. The program covers a wide range of topics, including molecular prevention, precision therapy, and patient-centered approaches. In addition, there are plenty of networking opportunities for participants to connect with peers and share ideas.

Arndt Vogel, Managing Senior Consultant and a Professor in the Department of Gastroenterology, Hepatology, and Endocrinology at Hannover Medical School, shared Highlights From ESMOGI25 on X:
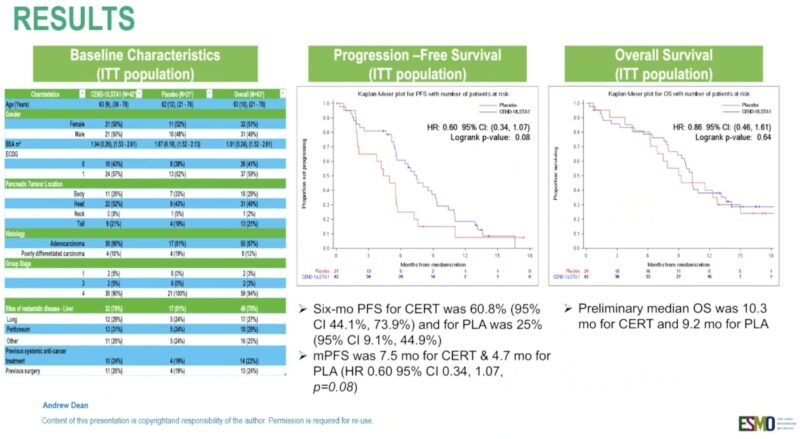
“Certepetide or plc added to GEM + nab-paclitaxel in mPDAC
AGITG ASCEND phase 2
ORR 33 vs 29%
mPFS 7.5 vd 4.5 mo
mOS10.3 vs 9 mo
Interesting MOA —> supposed to enhance uptake of anti-cancer drugs.”
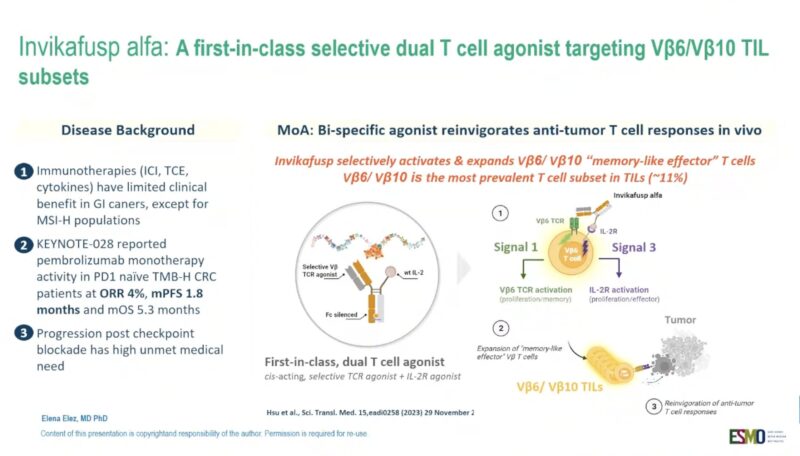
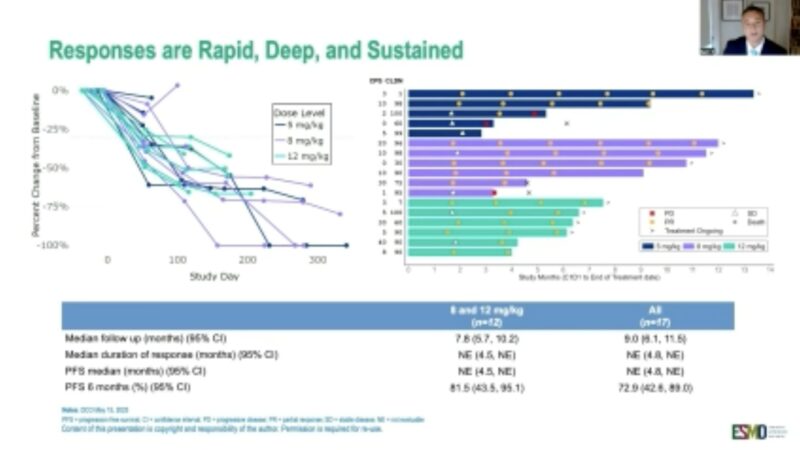
“Invikafusp alfa, a first-in-class TCR-beta chain-targeted bispecific antibody, in anti-PD(L)1-resistant, antigen-rich GI cancers
Phase I/II
60% MSI CRC
ORR: TMB-H GI 23%, TMB-H CRC: 25%
Interesting activity as monotherapy in subgroup of pretreated patients.”
“Givastomig, a novel claudin 18.2/4-1BB bispecific antibody, in combination with nivolumab and mFOLFOX in mGEC
Phase I/II
ORR 71%
Interesting activity.”
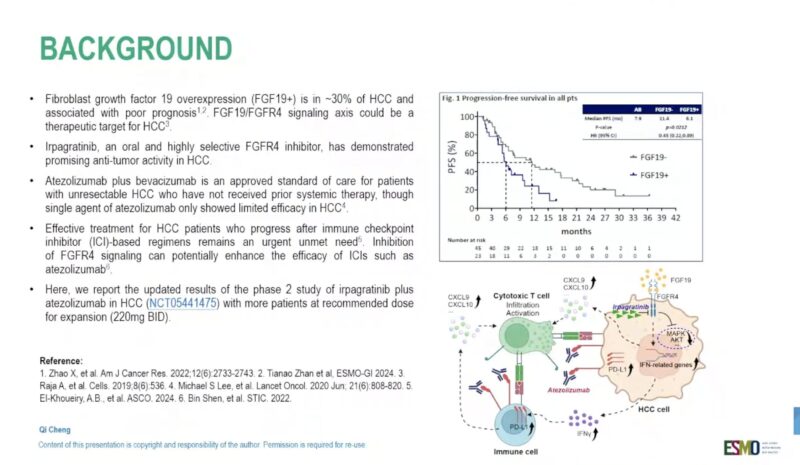
“Irpagratinib (ABSK-011) + atezolizumab in 1L naive and pretreated HCC with FGF19 overexpression
Updated results of phase II ABSK-011-201 study
ORR 50% vs 52%
mPFS 7.0 vs 8.3mo
Interesting activity, good safety profile.”
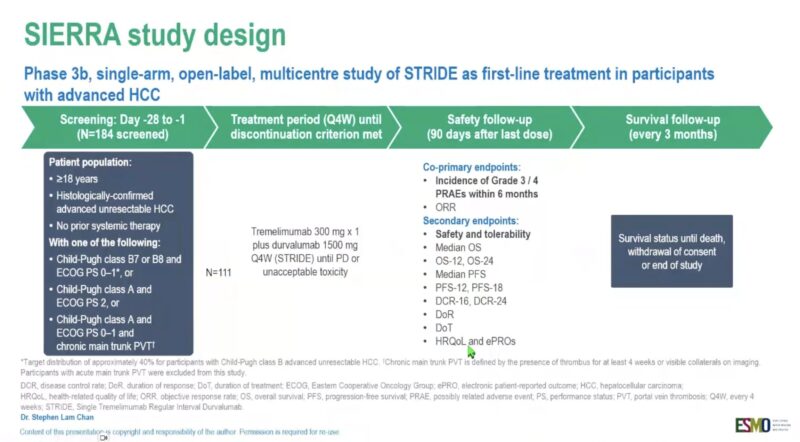
“Safety results for durvalumab and tremelimumab as 1L treatment for HCC with poor prognosis
SIERRA phase IIIb
short exposure in CB 7/8
higher PRAE in CP A, VP4
no new safety signals, but more efficacy data are needed.”
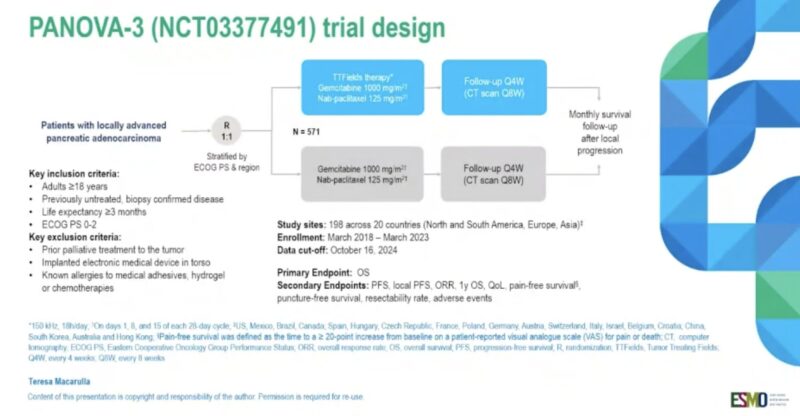
“Pain and QoL outcomes with TTFields therapy in patients with locally advanced PDAC
PANOVA-3 phase III
mPFS & mOS improved
Pain & QoL improved, less opioid use
Looking forward to see implementation in clinical practice.”
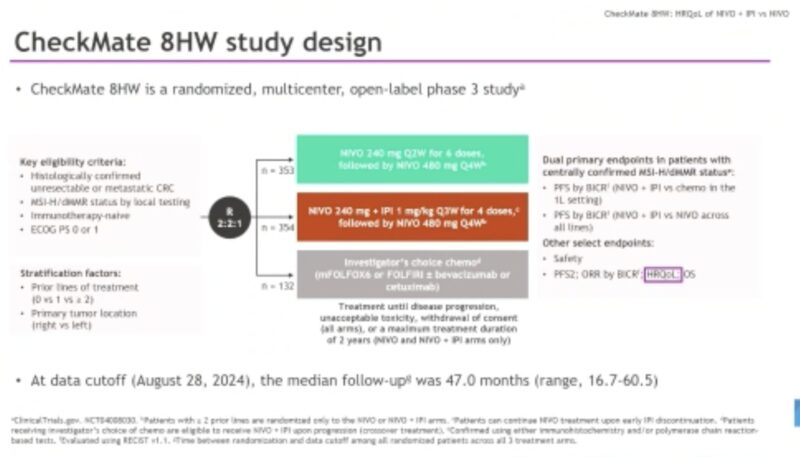
“NIVO + IPI vs NIVO for MSI-H/dMMR mCRC: Health-related quality of life analysis
CheckMate 8HW
mPFS clearly improved
HRQoL, less symptoms
Supports use of Nivo IPI as 1st line treatment in MSI CRC.”
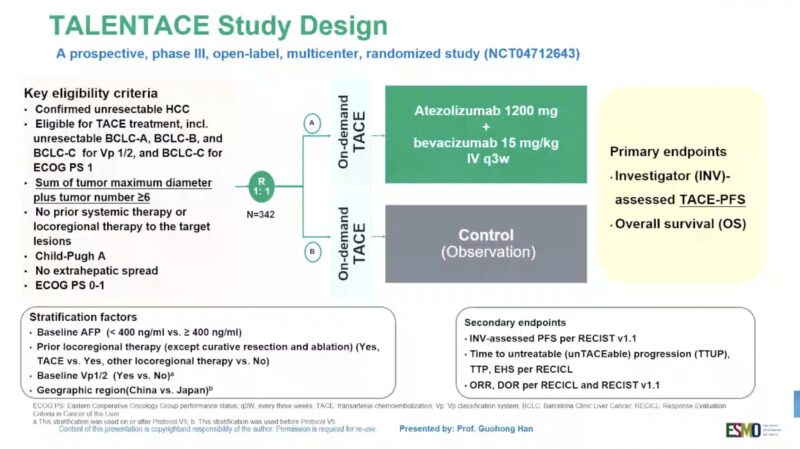
“On-demand TACE + with Atezo+Bev or TACE alone in intermediate-to-high burden HCC
TALENTACE phase III
ORR 49 vs 34%
mPFS 11.3 vs 7.03
mOS 34 vs 25 mo
In line with LEAP-12 & EMERALD-1, still no mature OS data, supportive”
“Overall Health-related quality of life and efficacy assessment in patients who discontinued due to TRAEs
CheckMate 9DW
Time to def. deterioration: 22.3 vs 0.5 mo
20% stopped due to TRAEs —> OS benefit maintained
Interesting analysis, supporting use of Nivo IPI.”

-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
