
Tafasitamab-cxix (Monjuvi) 2025 Updates: Uses in Cancer, Dosage, Side Effects, Expectations, and More
Tafasitamab-cxix is a targeted immunotherapy approved for certain types of aggressive B-cell lymphomas. As a monoclonal antibody that targets the CD19 protein on B-cells, it enhances the immune system’s ability to eliminate malignant cells. This article explores what tafasitamab-cxix is, how it works, its main uses in cancer care, recommended dosage, possible side effects, and what patients can expect during treatment.
Which company produced Tafasitamab-cxix?
Tafasitamab-cxix (Monjuvi) is produced by Incyte Corporation, a U.S.-based biopharmaceutical company known for developing targeted therapies in oncology and other serious diseases. Originally co-developed with MorphoSys, Incyte took full global rights to tafasitamab in 2024. The company is also known for other cancer treatments like Jakafi and Pemazyre.
How does Tafasitamab-cxix work?
Tafasitamab-cxix (Monjuvi) is a targeted immunotherapy designed to attack cancerous B-cells by binding to a protein called CD19, which is present on their surface. Once it attaches to CD19, it activates the immune system in several ways to eliminate the cancer cells.
It can cause apoptosis, which means it signals the cancer cell to die. It also boosts the immune response by recruiting natural killer (NK) cells to destroy the cell through a process called antibody-dependent cellular cytotoxicity (ADCC). Additionally, it helps bring in macrophages—immune cells that engulf and digest the cancer cells—in a process known as antibody-dependent cellular phagocytosis (ADCP).
Tafasitamab is specially modified to enhance these effects, making it more effective than standard antibodies in activating the immune system against lymphoma cells.
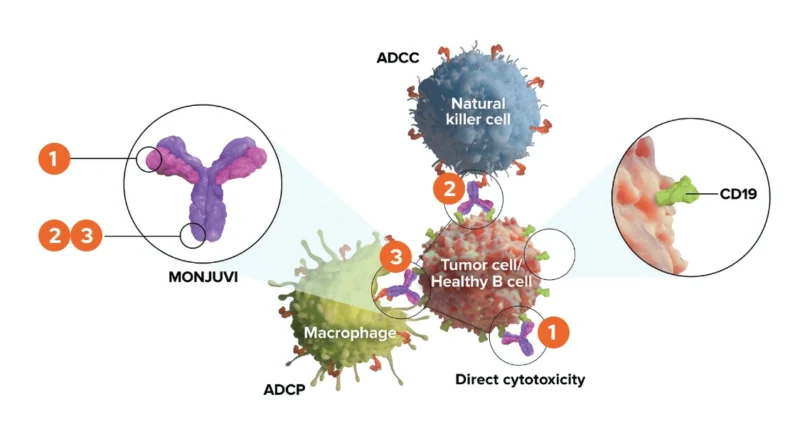
Photo from MONJUVI official website
What Cancers are treated with Monjuvi?
Monjuvi has gained FDA approvals for use in combination with other therapies to address relapsed or refractory forms of lymphoma.
In July 2020, the U.S. FDA granted accelerated approval to Monjuvi in combination with lenalidomide for adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who are not eligible for autologous stem cell transplant. This was the first FDA-approved second-line option in this setting, based on overall response rates. Continued approval may depend on confirmatory trials.
Later, in June 2025, the FDA approved Monjuvi in combination with rituximab and lenalidomide for adults with relapsed or refractory follicular lymphoma (FL).
What research is behind the approval?
The FDA’s 2020 accelerated approval of Monjuvi (tafasitamab-cxix) in combination with lenalidomide for relapsed or refractory DLBCL was based on results from the L-MIND trial (NCT02399085). This open-label, single-arm, multicenter study enrolled 81 patients who were not candidates for autologous stem cell transplant.
Patients received tafasitamab-cxix along with lenalidomide for up to 12 cycles, followed by tafasitamab monotherapy. The treatment showed encouraging efficacy, with an overall response rate (ORR) of 55%, including 37% complete responses and 18% partial responses. The median duration of response was 21.7 months.
You can find more information about approval on FDA official website.
The 2025 FDA approval of Monjuvi (tafasitamab-cxix) for relapsed or refractory follicular lymphoma (FL) is based on the results of the Phase 3 inMIND trial (NCT04680052), which were presented at the European Hematology Association (EHA) Congress 2025. This double-blind, placebo-controlled study evaluated the combination of tafasitamab, lenalidomide, and rituximab in patients with relapsed or refractory FL.
Involving over 540 patients, the trial showed that adding tafasitamab to the standard lenalidomide-rituximab regimen significantly improved progression-free survival (PFS). Median PFS reached 22.4 months in the tafasitamab arm compared to 13.9 months with placebo, translating to a 57% reduction in the risk of progression, relapse, or death (HR 0.43; p<0.0001). Benefits were seen across all subgroups, including patients with early relapse (POD24) and those resistant to prior anti-CD20 therapies.
The combination was well tolerated, with a safety profile consistent with expectations. The most common grade 3 or 4 adverse event was neutropenia, and overall serious side effects were similar between the treatment and placebo groups. These findings position tafasitamab plus lenalidomide and rituximab as a promising new option—and potential standard of care—for patients with relapsed or refractory follicular lymphoma.
You can also read about Immunotherapy for Lymphoma: Types, Success Rate, Side Effects and More on OncoDaily.
Tafasitamab-cxix Side Effects and Its Management
Tafasitamab-cxix, like many monoclonal antibodies, works by helping the immune system attack cancer cells, but this mechanism can also affect healthy cells and trigger side effects. While many patients tolerate the treatment well, it’s important to understand the potential side effects—both common and serious—as well as how they can be monitored and managed throughout therapy.
Common Side Effects
The most common side effects involve low blood counts—neutropenia, anemia, and thrombocytopenia—which can increase the risk of infection, fatigue, and bleeding. Other frequent symptoms include diarrhea, cough, decreased appetite, fever, swelling in the extremities, and respiratory infections. Fatigue is especially common and may persist throughout treatment.
Less Common and Serious Side effects
Less commonly, patients may develop infusion-related reactions, usually with the first few doses. These can present as chills, flushing, rash, shortness of breath, or high blood pressure. Such reactions are usually manageable with premedication or adjusting the infusion rate. Additional side effects such as joint or muscle aches, nausea, and mild hair thinning have also been reported but are less frequent.
Serious side effects are mainly related to bone marrow suppression, particularly severe neutropenia, which raises the risk of life-threatening infections. Although rare, fatal complications have occurred, often related to infection or disease progression. These risks underscore the importance of close monitoring.
Management of Side effects
Management of side effects includes regular blood tests before each cycle to detect low counts early. If problems are detected, treatment may be delayed or the dose reduced. Medications like G-CSF can support white blood cell recovery. Infusion reactions are handled with premedication and supportive care, while infection risks are reduced through hygiene, preventive antibiotics, and early treatment of symptoms.
With consistent monitoring and proactive management, most side effects of tafasitamab-cxix can be controlled, allowing patients to continue benefiting from treatment safely.

What is the Recommended Dosage of Tafasitamab-cxix?
Tafasitamab-cxix (Monjuvi) is available as a 200 mg vial of lyophilized powder. Its dosing varies based on the type of lymphoma being treated and is always given in combination with other medications.
Dose for Diffuse Large B-cell Lymphoma
For DLBCL, tafasitamab is used alongside lenalidomide in patients who have relapsed or refractory disease and are not eligible for stem cell transplant. In the first cycle, it is given on Days 1, 4, 8, 15, and 22. In cycles 2 and 3, it is administered weekly, and from cycle 4 onward, it is given every two weeks until disease progression or unacceptable side effects. Lenalidomide is taken orally at 25 mg daily for 21 days of each 28-day cycle, up to 12 cycles. After that, tafasitamab may continue alone. Dose adjustments for lenalidomide should follow its specific prescribing information, particularly for patients with kidney issues or clotting risks.
Dose for Follicular Lymphoma
For FL, tafasitamab is combined with both lenalidomide and rituximab. During the first three cycles, tafasitamab is given weekly, and from cycle 4 to 12, every two weeks. Lenalidomide is taken at 20 mg daily for 21 days of each cycle for up to 12 cycles. Rituximab is given weekly during the first cycle and then once per cycle for the next four cycles. Both lenalidomide and rituximab should be dosed according to their own prescribing guidelines, especially in patients with other medical conditions.
How is Tafasitamab-cxix administered?
Tafasitamab-cxix is given as an IV infusion after being reconstituted with sterile water and diluted in 0.9% saline. The infusion is prepared gently—without shaking—and visually checked for clarity.
Premedication (antihistamines or steroids) is recommended before the first three infusions to reduce the risk of infusion reactions. If no reactions occur, premedication may be skipped later. The first dose is infused slowly over 1.5–2.5 hours; subsequent doses over 1.5–2 hours.
The drug should not be mixed with other medications in the same IV line. Reconstituted or diluted solutions must be protected from light and should not be frozen or shaken. They can be stored at room temperature for up to 12 hours or refrigerated if needed.
What to Avoid During Tafasitamab-cxix Treatment?
During Monjuvi treatment, patients should avoid exposure to infections, as the drug can lower white blood cell counts and weaken the immune system. Infusion reactions may occur early in treatment, so premedication and monitoring are important. Pregnancy should be avoided due to potential harm to the fetus—effective birth control is required during treatment and for at least three months afterward. Regular blood tests help manage safety throughout therapy.
Tafasitamab-cxix effectiveness over time
Tafasitamab-cxix has shown lasting benefits in relapsed or refractory B-cell lymphomas. In DLBCL, it achieved a 55% response rate with a median response duration of nearly 21.7 months in the L-MIND trial. In follicular lymphoma, the inMIND trial showed it extended progression-free survival to 22.4 months when added to lenalidomide and rituximab, compared to 13.9 months without it. These results suggest tafasitamab provides durable responses in eligible patients.
Ongoing trials with Tafasitamab-cxix
The TaZA CLL trial (NCT05718869) is a Phase 2 study testing tafasitamab and zanubrutinib in newly diagnosed chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL). Led by City of Hope, the trial aims to assess safety and response. Patients receive both drugs—tafasitamab by IV and zanubrutinib orally—with regular monitoring. Researchers also study immune response, minimal residual disease, and treatment-related biomarkers.
The MD Anderson-led trial (NCT06434363) tests tafasitamab combined with AD-PluReceptor NK cells and lymphodepleting chemotherapy in patients with autoimmune diseases like systemic sclerosis and lupus (including lupus nephritis). Phase I focuses on safety and finding the optimal dose, while Phase II evaluates whether the treatment can control disease activity. Researchers also track immune recovery and response rates.
FAQ
What is Tafasitamab-cxix used for?
Tafasitamab-cxix (Monjuvi) is an FDA-approved immunotherapy used to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL).
How does Monjuvi work in the body?
Tafasitamab-cxix targets the CD19 protein on B-cells. By binding to this surface marker, it activates immune responses—including cell death, natural killer (NK) cell activity, and macrophage-mediated phagocytosis—leading to the destruction of cancerous B-cells.
What are the common side effects of Monjuvi ?
The most common side effects include low white blood cell counts (neutropenia), fatigue, fever, diarrhea, cough, and reduced appetite. Some patients also experience infusion-related reactions during the first few doses.
How is Tafasitamab-cxix administered?
Monjuvi is given as an intravenous (IV) infusion. It’s prepared by reconstituting the lyophilized powder and diluting it in saline. Premedication is usually recommended to minimize infusion-related reactions.
Can Tafasitamab-cxix be combined with other cancer treatments?
Yes, Monjuvi is used in combination with lenalidomide for DLBCL and with lenalidomide plus rituximab for follicular lymphoma.
How is Tafasitamab-cxix eliminated from the body?
Tafasitamab-cxix is metabolized like natural antibodies, breaking down into small peptides and amino acids through normal protein degradation pathways.
What is the half-life of Tafasitamab-cxix?
The half-life of tafasitamab-cxix is approximately 17 days, meaning it remains active in the body for about two and a half weeks.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
