
Kunal Jobanputra/researchgate.net
May 25, 2025, 14:50
Kunal Jobanputra Shares Key Clinical Studies from ASCO 2025
Kunal Jobanputra, Community Medical Oncologist at Tata Memorial Hospital, shared some studies from ASCO 2025:
Immunological approach to cancer interception by DNA plasmid vaccine
” ‘Immunological approach to cancer interception by DNA plasmid vaccine’ Phase IB study. INO-5401 encoding hTERT, PSMA and WT1, +/- IL12 and electroporation in healthy individuals with BRCA1/2 without cancer – Safety data so far.”
“Final CATNON results for anaplastic glioma without co-deletion:
-
Adjuvant TMZ boosts OS in IDH-mutant (HR 0.54, p<0.0001; median 12.5 yrs). No benefit in IDH-wt.
-
RT + 12 cycles adjuvant TMZ – standard for IDH-mt
-
Concurrent TMZ did not improve OS regardless of IDH status.”
“UCPVax in IDH1-wt GBM: to stimulate CD4+ helper T cell responses against telomerase (TERT)
Design: Phase IIa, 2-cohort; post-chemoradiation; Cohort A: vaccine alone; Cohort B: vaccine + 6 mo TMZ. Population: 61 patients (31 Cohort A: unmethylated MGMT; 30 Cohort B: 50% unmethylated). Outcomes: 83% (A) and 69% (B) had TERT-CD4+ response. 53% epitope spreading (57.7% A, 48% B)
OS 19.3 vs 12.8 mo with spread (P=0.03); RR 50% with spread vs 18.7% (P=0.05); safe, no serious AEs. Further trials for TERT vaccine in GBM. Effect of TMZ-lymphopenia on epitope spreading?”
RANO classification for extent of resection
“RANO classification for extent of resection. Population: Grade 2 IDH-mutant (379 astrocytoma, 349 oligodendroglioma) – who did not receive adjuvant Rx were allowed to study effects of resection.
-
Class 1 (Supramaximal): Resection beyond T2/FLAIR borders (0 cm³ remnant), 10-yr OS 97.5%.
-
Class 2 (Maximal): 0-5 cm³ remnant, 10-yr OS 82.2%.
-
Class 3 (Submaximal): 5-25 cm³ remnant, 10-yr OS 75%.
-
Class 4 (Minimal): >25 cm³ remnant, 10-yr OS 45.6%.”
Off-label use of T-DXd in Desmoplastic Small Round cell Tumor
“Off-label use of T-DXd in Desmoplastic Small Round cell Tumor, study from Memorial Sloan Kettering Cancer Center.
-
Studied HER2 by IHC (CB11 and 4B5) and also expression by RNA-seq in DSRCT
-
CB11 IHC showed 38/52 cases with > 10% HER2 staining and RNA-seq had third highest HER2 expression among pediatric solid tumors
-
Used in 16 patients with R/R DSRCT: 50% PR (8/16), 100% clinical benefit
-
8/8 had prior exposure to irinotecan
-
No response correlation with IHC/RNAseq.”
CARv3-TEAM-E to tackle challenge of heterogeneity with conventional CAR-T in GBM
“CARv3-TEAM-E to tackle challenge of heterogeneity with conventional CAR-T in GBM: T cells targeting EGFRvIII + T-cell-Engaging Antibody Molecules (TEAMs) against wild-type EGFR in recurrent GBM. Intraventricularly delivered CARv3-TEAM-E T cells were detected in the CSF and peripheral blood. Subsequent infusions – Ab development.”
“OASIS 4 ph3 trial
-
Elinzanetant: a dual NK1/NK3 antagonist
-
For vasomotor symptoms in HR+ breast cancer patients on adjuvant ET
-
Elinzanetant reduced vasomotor symp frequency at week 4 (-6.5 vs -3.0, p<0.0001) and week 12 (-7.8 vs -4.2, p<0.0001).”
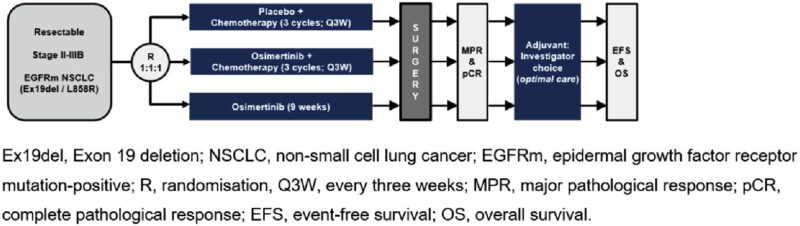
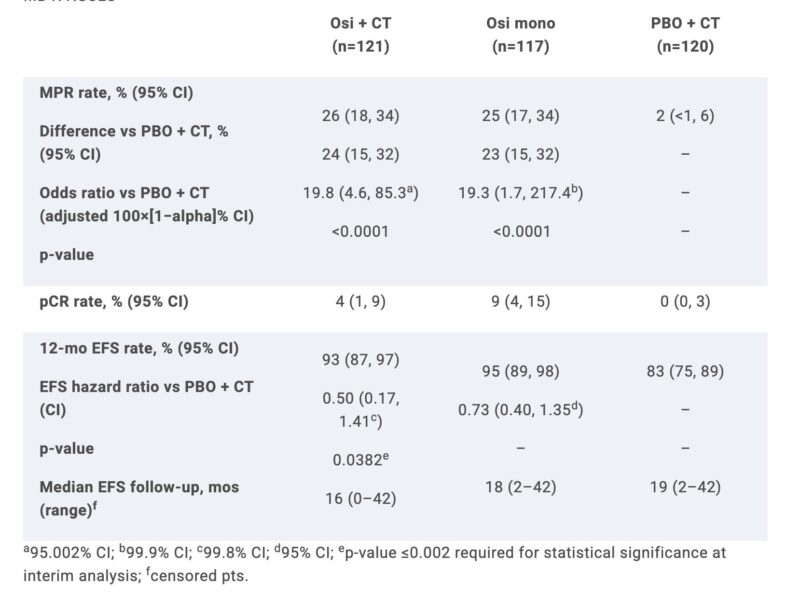
“NeoADAURA: EGFRm (Ex19del/L858R) stg II–IIIB (AJCC 8th ed)
1:1:1 to
neoadj osi 80 mg QD (≥9 weeks) + CT
Osi monotherapy 80 mg QD (≥9 weeks) or
Placebo + CT
Osi/PBO + CT: double blind; osi mono: open label, sponsor blind. Adj osi was offered to all patients who completed surgery. Primary endpoint: MPR. Neoadjuvant Osi +/- CT showed improved MPR vs CT alone.”
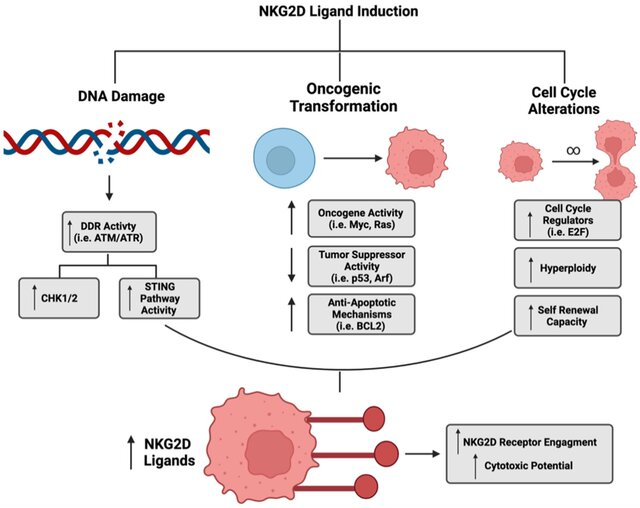
“γδ T cells for GBM
Ph1 trial used γδ T cells + TMZ, targeting NKG2D-L via DDR pathway. γδ T cells engineered for TMZ resistance with MGMT
=13, got 1, 3, or 6 doses (1×10^7 cells) in resection cavity + TMZ in Stupp regimen.
No DLTs/CRS/ICANS. FU 16.9 mo.
mPFS: 9.9m in all-comers, 14.0m (multi-dose)
γδ T cells target stress-induced GBM ligands for better control
NKG2D-L induction diagnosis.”
Camrelizumab and apatinib in advanced chordoma
“Camrelizumab + apatinib in advanced chordoma.
Phase 2
n=33 patients
mFU 15 mo.
ORR 21.2% PR, 6-mo DCR 85.2%. Choi: 48.5% PR, DCR 77.7%.
Median PFS: 18.1 mo (RECIST), 15.3 mo (Choi).
CDKN2A deletion correlated with worse outcomes.”
Final analysis of adjuvant Nivo in pateints with resected EC/GEJ post CRT
“Final analysis of adjuvant Nivo in pateints with resected EC/GEJ post CRT – Sx with residual path disease
mFU = 78 months!
mDFS = 21.8vs 10.8 mo
HR DFS = 0.76 [95% CI 0.63–0.91]
mOS = 51.7 vs 35.3 mo
HR OS =0.85 [95.87% CI 0.70–1.04 ? (expected HR was 0.73)
5-yr OS = 46% vs 41%
Subsequent IO : 5% vs 15%
KM curves awaited!”
Romiplostim vs Placebo in chemotherapy-induced thrombocytopenia
“First global ph3 trial to study Romiplostim vs Placebo in chemotherapy-induced thrombocytopenia
Phase 3 RCT, 2:1 ROMI vs PBO
n=65 on Oxaliplatin based Cth (75% colorectal, 13% gastroesophageal, 12% pancreatic), Plt ≤85×10⁹/L on day 1 of Cth
Weekly SC ROMI (2–10 μg/kg) for 3 cycles
Primary: No CIT-induced dose modification in cycles 2 or 3
Secondary: Plt nadir, time to Plt response, safety
84% ROMI vs 36% PBO met primary endpoint (OR 10.2, P<0.001)
Plt nadir : 87 vs 58×10⁹/L (P=0.005); faster Plt response (1.1 vs 2.1 wks, P<0.001)
Further data on survival outcomes, side-effects on longer follow-up needed.”
More posts featuring Kunal Jobanputra.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
Sep 5, 2025, 11:17
Sep 5, 2025, 10:43
Sep 5, 2025, 10:29
