
Pemigatinib (Pemazyre): What patients need to know?
Pemigatinib is a targeted cancer medication designed to treat specific types of cancers caused by changes in the FGFR genes. This drug has been approved by the U.S. Food and Drug Administration (FDA) for treating advanced bile duct cancer (cholangiocarcinoma) and a rare type of blood cancer known as myeloid/lymphoid neoplasms with FGFR1 rearrangement. By targeting these unique genetic changes in cancer cells, pemigatinib offers a personalized treatment option that can slow or stop tumor growth.
What Is Pemigatinib and How Does It Work?
Pemigatinib, marketed as Pemazyre®, belongs to a class of drugs called FGFR inhibitors. FGFR stands for fibroblast growth factor receptor, a group of proteins on the surface of cells that help regulate cell growth and survival. In some cancers, including certain bile duct cancers and blood cancers, these receptors become abnormal due to genetic mutations or rearrangements. This abnormal FGFR activity causes cancer cells to grow uncontrollably.
Pemigatinib works by blocking these faulty FGFR proteins, specifically FGFR1, FGFR2, and FGFR3. By stopping the signals that promote cancer cell growth, the drug helps to slow down or stop tumor progression. Because it targets a specific genetic change, pemigatinib is considered a precision medicine—it treats the cancer based on the tumor’s unique genetic profile.
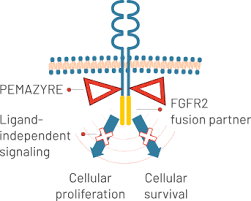
Photo form the official PEMAZYRE® website
What Cancers Does Pemigatinib Treat?
Pemigatinib is FDA-approved for adults with cholangiocarcinoma that cannot be removed by surgery or has spread to other parts of the body (metastatic) and whose tumors have FGFR2 gene fusions or rearrangements. It is also approved for a rare aggressive blood cancer called relapsed or refractory myeloid/lymphoid neoplasms (MLNs) with FGFR1 rearrangement.
Researchers are also studying pemigatinib for other cancers that have FGFR gene changes, including pancreatic cancer and certain types of soft tissue sarcomas, to see if it could benefit patients with these tumors as well.
What Is a Clinical Trial and Why Does It Matter?
A clinical trial is a research study designed to test new drugs and treatments in patients to determine their safety and effectiveness. Before Pemazyre was approved, it went through multiple phases of clinical trials to assess how well it worked, what side effects it caused, and whether it was better than existing treatments. Clinical trials are essential because they provide scientific evidence that a drug can help patients while ensuring it is safe for widespread use.

What Does FDA Approval Mean?
When a drug receives FDA approval, it means that after rigorous testing in clinical trials, it has been shown to be both safe and effective for treating a specific condition. This approval makes the drug widely available for doctors to prescribe and helps patients access new, cutting-edge treatments sooner.
How Effective Is Pemigatinib?
The approval of pemigatinib for cholangiocarcinoma was based on a clinical study called FIGHT-202. This study showed that about 36% of patients with FGFR2 alterations experienced tumor shrinkage after treatment. Remarkably, some patients had complete disappearance of their tumors. On average, these responses lasted about 9 months, with many patients maintaining benefits beyond six months.
For the blood cancer indication, the FIGHT-203 study showed even more promising results. Nearly 80% of patients in the chronic phase of disease achieved a complete response, meaning no signs of cancer were detected after treatment. This response usually happened within about three and a half months.
These results bring hope to patients with few other treatment options, showing that pemigatinib can provide meaningful control over cancer growth.

You can read about Cholangiocarcinoma: Latest Risk Factors, Symptoms, Diagnosis, and 2025 Advanced Treatment Options for Bile Duct Cancer on OncoDaily.
Pemigatinib side effects and management
Pemazyre (pemigatinib) is a targeted therapy used to treat certain cancers with FGFR gene changes. Like many cancer treatments, it can cause side effects. Understanding these effects and knowing how to manage them can help patients feel more in control and continue treatment safely.
Common Side Effects
The most common side effect is high levels of phosphate in the blood, known as hyperphosphatemia. Other frequently reported issues include brittle or discolored nails, hair loss, mouth sores, diarrhea, dry eyes, fatigue, and skin rashes. Some patients also experience stomach pain, dry mouth, nosebleeds, dizziness, or changes in vision such as blurred sight or fluid buildup behind the retina. These side effects are usually manageable but should be discussed with your care team.
Less Common Side Effects
Less common side effects may include low levels of phosphate, white blood cells, red blood cells, or platelets. Some patients may also develop higher liver enzyme levels, which could be a sign of liver irritation. Swelling, poor appetite, dry skin, upset stomach, back pain, and nausea may also occur, though less frequently. These effects can be more serious and may require adjusting the dose or pausing treatment temporarily.
Managing Side Effects
Most side effects can be managed with close monitoring and supportive care. High phosphate levels are typically treated with a low-phosphate diet and, if needed, phosphate-lowering medications. Vision changes should be reported right away, and regular eye exams are recommended during treatment. Mild symptoms like fatigue, diarrhea, and mouth sores can often be relieved with rest, hydration, and special oral care. Hair loss and nail problems can be upsetting but are usually reversible after treatment ends. Blood counts should be checked regularly, and your doctor may adjust your treatment if changes are detected.
It’s important to tell your healthcare team about any new or worsening symptoms. Early recognition and management of side effects can help reduce discomfort and allow you to stay on treatment safely and effectively.
Recommended Dosage of Pemigatinib
Pemigatinib (brand name Pemazyre®) is a targeted cancer treatment that comes in tablet form. It is used to treat two types of cancer: advanced bile duct cancer (cholangiocarcinoma) with FGFR2 gene changes, and a rare type of blood cancer called myeloid/lymphoid neoplasms (MLNs) with FGFR1 gene changes. For bile duct cancer, the usual dose is 13.5 mg taken once a day for 14 days, followed by 7 days off. This 21-day cycle repeats for as long as the treatment is working and side effects are manageable. For blood cancers (MLNs), the same dose (13.5 mg daily) is taken continuously, without breaks.
If side effects occur, your doctor may lower the dose to 9 mg or 4.5 mg per day. If certain side effects become severe—like vision problems or high phosphate levels—treatment may need to be paused or adjusted. If the lowest dose isn’t well tolerated, the treatment might be stopped.
How is Pemigatinib Taken?
Pemigatinib is taken by mouth once a day, with or without food, at the same time each day. The tablets must be swallowed whole—do not crush, chew, split, or dissolve them. If you miss a dose and it’s been more than four hours, just skip it and take your next dose at the usual time. Do not take an extra dose to make up for a missed one. If you vomit after taking it, wait and take your next dose at the regular time.
Store the tablets at room temperature, between 20–25°C (68–77°F), though short changes between 15–30°C (59–86°F) are okay. Always follow your doctor’s instructions and let them know if you have any side effects or questions during treatment.
What Should Patients Avoid During Treatment?
To ensure safety, patients should avoid grapefruit or grapefruit juice, as these can increase drug levels and side effects. It’s also important to inform your doctor about any other medications or supplements you are taking to avoid harmful interactions. Because pemigatinib can cause dizziness or blurred vision, caution is advised when driving or operating machinery. Additionally, live vaccines should be avoided during treatment to reduce infection risk.
You can read more about Liver Cancer on OncoDaily.
What Is the Long-Term Outlook with Pemigatinib?
Pemigatinib offers many patients lasting control of their cancer, sometimes for several months or longer. While it may not cure advanced cancers, it helps manage the disease, slow progression, and improve symptoms. Patients usually continue treatment as long as it remains effective and side effects are tolerable.
If the cancer progresses, your doctor may recommend other therapies or clinical trials. Ongoing research aims to improve the effectiveness and safety of pemigatinib and its use in combination with other treatments.
Real-World Use and Looking Ahead
Beyond clinical trials, pemigatinib is being used by patients worldwide with FGFR-altered cancers, and ongoing studies continue to evaluate its long-term benefits and safety in everyday medical practice.
Scientists are hopeful that pemigatinib, especially when combined with immunotherapy or other targeted drugs, will help more patients with different cancer types in the future. This drug represents an important step forward in personalized cancer treatment, bringing hope to patients who need new, effective options.
What Are Ongoing Clinical Trials?
Scientists continue to explore pemigatinib in several clinical trials. One study is testing pemigatinib in pancreatic cancer patients with FGFR changes to see if it can slow tumor growth. Other trials are investigating combining pemigatinib with immunotherapy drugs like durvalumab and retifanlimab in cancers like cholangiocarcinoma and soft tissue sarcomas. These combination therapies may improve treatment outcomes and offer new options for patients in the future.
Pemigatinib is being studied in several clinical trials to see if it can help treat more types of cancer with FGFR gene changes. One ongoing trial is testing pemigatinib alone in people with advanced pancreatic cancer to see how well it slows tumor growth and how safe it is.
Another study is combining pemigatinib with durvalumab, an immunotherapy, in patients with bile duct cancer that has returned after treatment. This combination aims to see if using both drugs together is more effective.
A third trial is looking at pemigatinib with retifanlimab, another type of immunotherapy, in patients with advanced liposarcoma (a rare soft tissue cancer). These studies may help expand the use of pemigatinib to more cancers in the future.
If you’re a healthcare provider, access the professional version here.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
