Neurological Side Effects of Chemotherapy
Cancer represents one of the most pernicious public health problems with a high mortality rate among patients worldwide. Chemotherapy is one of the major therapeutic approaches for the treatment of various malignancies. Chemotherapy, a cornerstone in cancer treatment since the 20th century, paradoxically introduces severe side effects that diminish treatment efficacy and patient well-being. The chemotherapy prescription approaches rely on various elements, including the cancer’s type, the cancer’s stage, the patient’s age, the patient’s general health status, the other concurrent health issues, and the history of receiving chemotherapies. Understanding the mechanisms underlying chemotherapy-induced neurotoxicity is crucial for developing neuroprotective drugs, alleviating symptoms, and enhancing treatment outcomes.

Photo: Depositphotos
This article delves into the mechanisms of neurological side effects of chemotherapy , focusing on its effects on the peripheral and central nervous systems, encompassing neuropathic pain, chemobrain, and finally outlining the management of the patient that needs a multi discipline approach.
What is Chemotherapy-Induced Neurotoxicity?
Chemotherapy-induced neurotoxicity refers to the adverse effects of chemotherapeutic agents on the nervous system, significantly impacting cancer patients’ quality of life. It is broadly classified into central nervous system (CNS) toxicity, affecting the brain and spinal cord, and peripheral nervous system (PNS) toxicity, impacting peripheral nerves. CNS toxicity can manifest as cognitive impairment (“chemobrain”), seizures, or encephalopathy, while PNS toxicity primarily presents as chemotherapy-induced peripheral neuropathy (CIPN), characterized by neuropathic pain, sensory deficits, and motor impairments.
Which Chemotherapeutic Agents Most Commonly Induce Neurotoxicity?
Several chemotherapeutic agents are commonly implicated in neurotoxicity. Platinum compounds (cisplatin, oxaliplatin, carboplatin) are notorious for causing CIPN and can also contribute to CNS toxicity. Taxanes (paclitaxel, docetaxel) and vinca alkaloids (vincristine, vinblastine) are also frequent culprits in CIPN. Proteasome inhibitors (bortezomib) and immunomodulatory drugs (thalidomide) can also induce neurotoxic effects.
The mechanisms underlying chemotherapy-induced neurotoxicity are multifactorial. Direct neuronal damage occurs through various pathways. Platinum drugs can form DNA adducts, disrupting DNA replication and leading to cell death.
Microtubule-targeting agents like taxanes and vinca alkaloids disrupt axonal transport and neuronal structure. Mitochondrial dysfunction, leading to oxidative stress and impaired energy production, is another crucial mechanism. Neuroinflammation, involving the release of pro-inflammatory cytokines and activation of glial cells, contributes to neuronal damage and blood-brain barrier (BBB) disruption. Disruption of synaptic plasticity, affecting learning and memory, is also observed. Recognizing and understanding these mechanisms is crucial for developing strategies to prevent or mitigate neurotoxic side effects, thereby improving cancer treatment outcomes and patients’ quality of life.
Chemotherapy-Induced Peripheral Neuropathy (CIPN)
The peripheral nervous system(PNS) is a common target for the neurotoxicity of several conventional chemotherapy drugs. This is mostly because of the less efficient blood–nervous system barrier in the PNS, namely at the dorsal root ganglia level, which allows easy access to nerve fibers and neurons (Guido Cavaletti et al, 2015) [8]. Direct toxic effects of antineoplastic drugs upon peripheral neurons are considerable, but also indirect effects contribute, mainly due to inflammatory reactions, leading to the development of chemotherapy-induced peripheral neuropathy (Halina Was et al, Front Pharmacol. 2022). The conventional drugs associated with chemotherapy-induced peripheral neurotoxicity(CIPN) are platinum compounds, taxanes, vinca alkaloids, epothilones, proteasome inhibitors, and thalidomide (Cavaletti, Alberti, & Marmiroli, ASCO 2015).
As chemotherapeutic drugs differ in their mechanisms of action, they affect the peripheral nervous system in various ways, leading to variable clinical features. CIPN manifests with sensory symptoms like numbness, tingling, and burning pain, often in a “stocking-and-glove” distribution. Motor symptoms, such as muscle weakness, and autonomic dysfunction can also occur, particularly with vincristine.
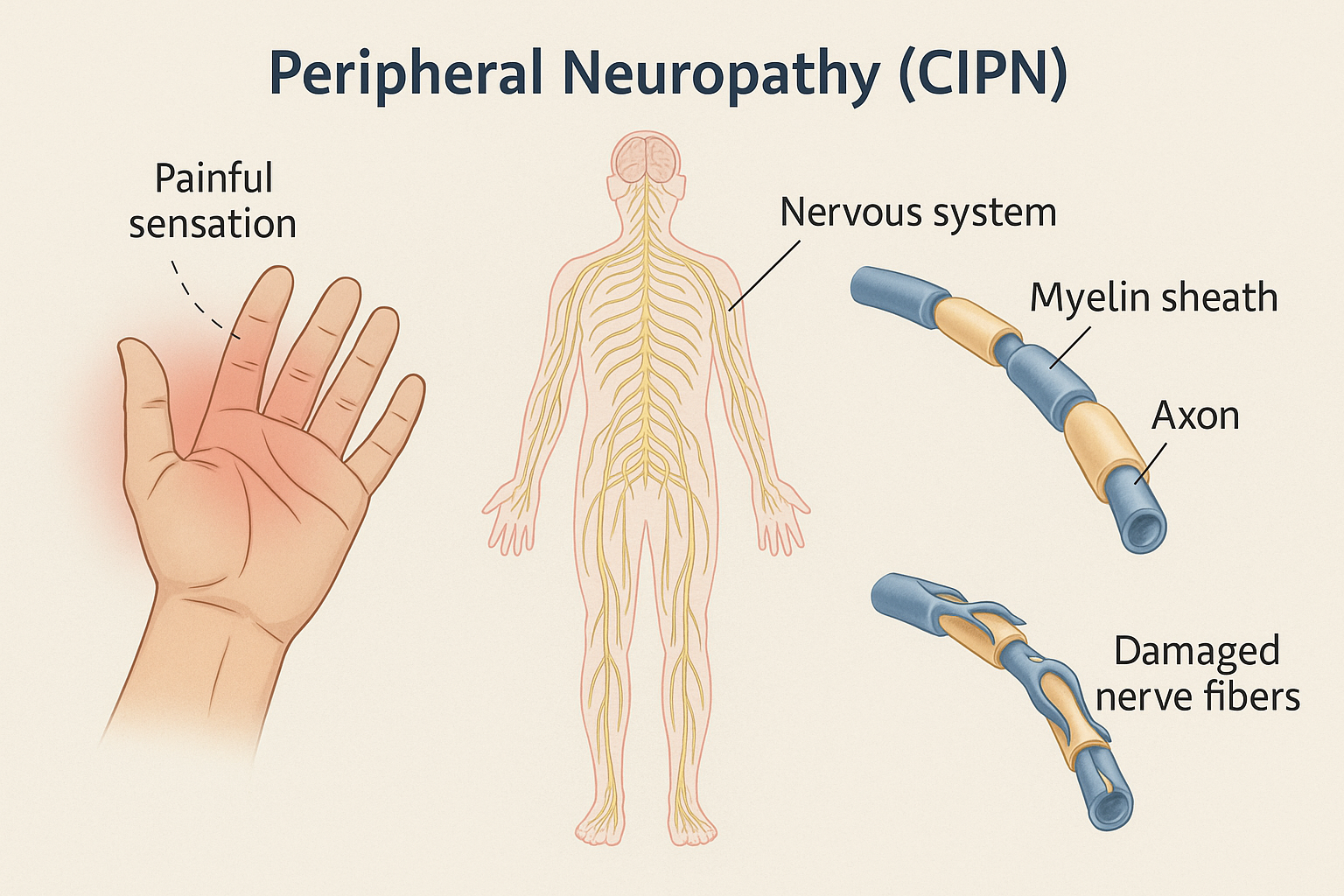
CIPN can be acute, appearing shortly after drug infusion, or chronic, persisting long after treatment completion. Acute CIPN is often characterized by transient symptoms such as muscle tightness, cramps, and paresthesias, particularly associated with oxaliplatin. These symptoms are often exacerbated by cold temperatures. In contrast, chronic CIPN involves more persistent and potentially irreversible nerve damage, leading to sensory deficits, neuropathic pain, and motor impairments. Chronic CIPN involves structural damage to neurons, mitochondrial dysfunction, and neuroinflammation, associated with platinum-based agents like cisplatin, carboplatin, proteasome inhibitors like bortezomib and thalidomide analogs, taxanes, including paclitaxel and docetaxel. Chronic symptoms can result in dose interruptions, subtherapeutic dosing, or even discontinuation of chemotherapy (Halina Was et al, Front Pharmacol. 2022).
What Is Chemobrain? Understanding Central Neurotoxicity and Cognitive Impairment
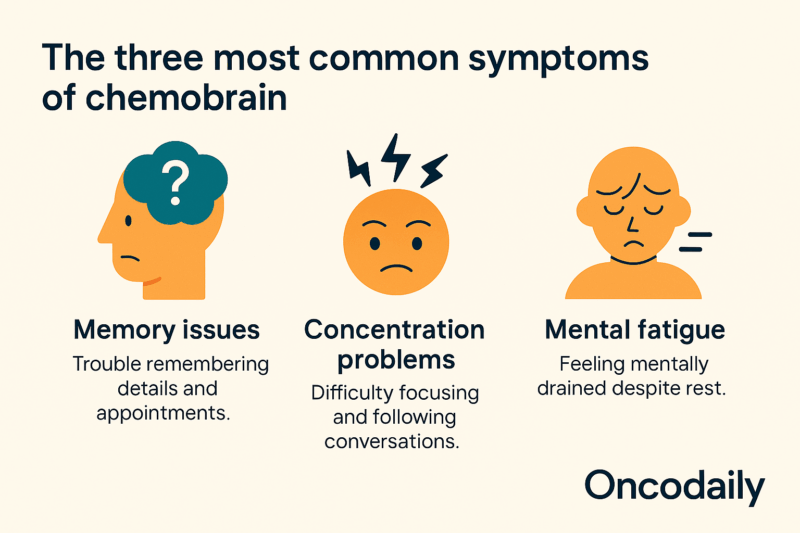
Chemotherapy drugs can induce severe side effects in the central nervous system (CNS), leading to conditions like “chemobrain”. Chemobrain, or chemotherapy-related cognitive impairment (CRCI), is a distressing side effect experienced by many cancer survivors. It is characterized by a range of cognitive deficits, including memory deficits, attention impairment, executive dysfunction, and mood changes, all impacting daily functioning and psychosocial well-being.
Clinically, chemobrain presents with difficulties in learning and retaining new information, reduced concentration, impaired multitasking, and challenges in decision-making. Mood changes, such as anxiety, depression, and increased irritability, often accompany these cognitive impairments. These symptoms can significantly affect a person’s ability to perform daily tasks, maintain employment, and engage in social activities.
Photo: Depositphotos
How Often Do Cancer Patients Experience Chemobrain?
Epidemiologically, chemobrain is most noticeable in breast cancer patients, with frequencies reaching up to 80%, though it also affects individuals with lung cancer, CNS malignancies, testicular cancer, and hematologic malignancies. Older adults are at higher risk, as receiving higher cumulative doses of chemotherapeutic agents. Several neurobiological mechanisms contribute to chemobrain, including blood-brain barrier (BBB) disruption, neuroinflammation, glial activation, synaptic plasticity impairment, and mitochondrial dysfunction.
Chemotherapeutic agents can cross the BBB, directly affecting microglia and astrocytes, inducing pathological gliosis and neuroinflammation. These drugs, including cisplatin and cyclophosphamide, can impair neurogenesis and disrupt neural network dynamics (Halina Was et al, Front Pharmacol. 2022)
Neuroimaging studies reveal alterations in the connectivity of key brain networks in chemobrain patients. Alterations in the default mode network, central executive network, and dorsal attention network connectivity have been reported, correlating with cognitive decline. These changes reflect impaired neural efficiency and reduced cognitive reserve. Understanding the definition, risk factors, mechanisms, and impact of chemobrain is essential for developing effective interventions and improving the quality of life for cancer survivors (Alan Umfress et al, ACS Chem Neuroscience 2021)
How Do Neurological Side Effects of Chemotherapy Manifest in Patients?
As it discussed before, chemotherapeutic agents affect both the peripheral(CIPN) and the central nervous system(Chemobrain). CIPN develops as a glove and stocking neuropathy, although in more severe cases it can spread proximally affecting most of the limbs. Whilst it is predominantly a sensory neuropathy, autonomic function can also be affected, as can fine motor function and proprioception, with evidence for loss of sensory fibres and reduced intraepidermal nerve fibre density (IENFD). Sensory dysfunction is wide ranging, with both positive and negative sensory signs, some of which are more often associated with particular types of chemotherapy e.g, cold hypersensitivity during platinum-based therapy.
Neuropathic descriptors such as burning, and shooting are often used, along with numbness and paraesthesia, although pain is not always a presenting feature. Other symptoms include mechanical allodynia(clothes on skin are painful), hyperalgesia, dysaesthesia, inability to detect thermal changes, etc. CIPN can persist for many years, with a detailed assessment of long term survivors of childhood cancers finding ~48% of individuals having some evidence of neuropathy: predominantly sensory dysfunction and reduced quality of life (Lesley A Colvin, Pain 2020).
Chemotherapy-related cognitive impairment(CRCI) symptoms are typically subtle and encompass memory impairments, learning difficulties, executive function deficits, reduced concentration, attention issues, and slower processing speed. Common difficulties reported by cancer survivors include experiencing mental ‘cloudiness’ or ‘fog,’ difficulty recalling words and names, challenges in maintaining focus, reduced ability to learn new information, difficulties in managing daily activities, and impaired multitasking capabilities (Mika Miyashita, Asia Pac J Oncol Nurs. 2023).
What Are the Diagnostic and Monitoring Tools for Neurological Side Effects of Chemotherapy?
Diagnostic tools are crucial for physicians to accurately identify and manage neurological side effects of chemotherapy. While systemic chemotherapy remains a cornerstone of cancer treatment, its neurotoxic effects can significantly impair a patient’s quality of life (Cavaletti, Alberti, & Marmiroli, ASCO 2015). Early and precise diagnosis is essential to mitigate long-term neurological dysfunction.
Clinical examination and patient history play a vital role in identifying potential neurotoxicity. Neurophysiological tests, such as nerve conduction studies and electromyography, can assess peripheral nerve function, while neuroimaging modalities like MRI, functional MRI, and diffusion tensor imaging can reveal CNS effects. Some tests are significant to give them a closer look.
Photo: Depositphotos
Clinical Grading Scales and Patient-Reported Tools
Commonly used clinical grading scales include the National Cancer Institute – Common Criteria for Adverse Events (NCI-CTC) and the Total Neuropathy Score (TNS), both of which are validated for monitoring and diagnosing chemotherapy-induced peripheral neuropathy (CIPN). Patient-reported outcome measures such as the Patient Neurotoxicity Questionnaire (PNQ) and the EORTC QLQ-CIPN20 questionnaire are also widely used to assess symptom severity and functional impairment.
Nerve Excitability Assessment
This method uses threshold tracking techniques to assess ion channels, pumps, and exchangers in large myelinated axons, offering greater sensitivity than conventional nerve conduction studies. Nerve excitability studies can detect early changes before axonal loss occurs, making them a valuable biomarker for early diagnosis and risk prediction in patients, especially those treated with oxaliplatin.
Quantitative Sensory Testing (QST) and Stocking and Glove Distribution Testing (SGDT)
QST and SGDT are used to assess sensory disturbances, such as pinprick and cold sensation, which can help in early diagnosis and monitoring of CIPN.
Laser Doppler Imager (LDI) FLARE Technique
The LDI FLARE technique is a novel diagnostic tool that measures small fiber function and has shown higher sensitivity in confirming CIPN compared to standard methods like nerve conduction studies or vibration perception threshold. It correlates well with patient-reported symptom severity and can detect early changes in small fiber function.
Conventional Nerve Conduction Studies
While these remain a standard in clinical practice, they are less sensitive to early or small fiber changes and are best used in conjunction with other methods.
MRI is the cornerstone for diagnosing central neurotoxicity from chemotherapy. It can reveal both nonspecific and specific patterns, such as white matter changes, toxic leukoencephalopathy, and reversible diffusion-weighted imaging (DWI) abnormalities. Certain agents (e.g., methotrexate, 5-FU, capecitabine) may produce distinctive MRI findings, including symmetrical periventricular DWI changes and involvement of the corpus callosum or basal ganglia. Recognizing these MRI patterns is essential for distinguishing neurotoxicity from other conditions such as metastases or paraneoplastic syndromes, enabling timely intervention and improved outcomes(Mina F G Isaac, Rugaiyah Al Khatib & Chi Long Ho, Insights Imaging 2024).
Clinical Management and Novel Therapeutic Strategies for Neurological Side Effects of Chemotherapy
While advancements in cancer treatment have increased survival rates, the neurological side effects of chemotherapy, including cognitive impairment and peripheral neuropathy, are becoming increasingly recognized. Current management strategies encompass a range of approaches, from pharmacological interventions aimed at symptomatic relief to experimental therapies focused on neuroprotection.
Pharmacological treatments often target the symptoms of neurotoxicity, such as neuropathic pain in the context of chemotherapy-induced peripheral neuropathy(CIPN). The 2020 ASCO guideline update does not recommend any specific agents for the treatment of CIPN other than the potential limited benefit of duloxetine for painful CIPN.
Several experimental neuroprotective drugs are under investigation, including those that target specific pathways involved in neuronal damage and repair mechanisms. These agents aim to prevent or reduce the severity of neurotoxic effects by directly protecting nerve cells from chemotherapy-induced injury. Non-pharmacological interventions, including physical and occupational therapy, play a crucial role in helping patients manage functional deficits. At the moment, no standard of care is available for chemobrain and treatment options are limited.
A reasoned approach may consist of a combination of strategies including reassuring the patient, treating comorbidities, encouraging physical activity and providing behavior interventions and cognitive training. Pharmacological strategies may include central nervous system stimulants (e.g., methylphenidate and modafinil), antidementia drugs (e.g., donepezil, memantine, and ginkgo biloba) but their efficacy in randomized clinical trials has not been yet established and their use in clinical setting remains limited (Paola Alberti et al, Cancers (Basel) 2022).
Emerging research explores experimental therapies, including anti-inflammatory drugs and antioxidants (Vitamin E), to combat the mechanisms driving neurotoxicity. Furthermore, lifestyle factors such as exercise, nutrition, and mental health support are increasingly recognized for their potential to improve outcomes for cancer survivors. Integrative approaches that combine conventional treatments with complementary therapies are also gaining attention. A collaborative effort between patients and physicians is essential to recognize early signs of neurotoxicity and prevent irreversible damage, ultimately improving the quality of survival for cancer patients.
Before initiating any treatment, it is essential to consult both your oncologist and neurologist ensure safe and appropriate management, minimizing potential risks to your health.
Bridging Oncology and Neurology
The complex landscape of cancer treatment necessitates a collaborative approach between oncologists and neurologists to effectively manage neurological side effects of chemotherapy. Neurologists play a vital role in the diagnosis, differential diagnosis, and management of these neurotoxicities. Their expertise is essential for identifying the specific neurological syndromes arising from chemotherapy, distinguishing them from other potential causes, and implementing appropriate treatment strategies. Conversely, oncologists are primarily focused on balancing the efficacy of cancer treatment with the potential risk of neurotoxicity. This requires a deep understanding of the neurotoxic potential of different chemotherapeutic agents and the ability to modify treatment strategies to minimize neurological damage.
The development of joint protocols and referral pathways is crucial for streamlining patient care. Multidisciplinary clinics, where neurologists and oncologists work side-by-side, go as successful care models. This collaborative care yields numerous benefits, including improved symptom control, enhanced treatment adherence, and ultimately, a better quality of life for patients.
To further optimize patient outcomes, increased education for both oncologists and neurologists regarding side effects of chemotherapy is essential. Furthermore, collaborative research efforts are needed to elucidate the underlying mechanisms of neurotoxicity and develop effective preventative and therapeutic strategies. Finally, the development of standardized guidelines for the management of these complications will ensure consistent and optimal care for all cancer patients.
You Can Also Read Medulloblastoma: Breakthroughs in Types, Symptoms, Diagnosis, Treatment, and 2025 Advances in Care by Oncodaily
You Can Also Watch Professor Etienne Brain | Leading Advances in Breast Cancer and Geriatric Oncology by Oncodaily
Conclusion
Neurological side effects of chemotherapy poses challenges for cancer treatment, greatly affecting how patients live and function. Even with its critical role for the controlling of malignancies, chemotherapy can lead to irreversible neurological damage which includes peripheral neuropathy and also cognitive impairment because such damage can severely impact on long-term survivorship. Because neurologists, oncologists, and rehabilitation specialists accurately diagnose, monitor, and treat neurotoxic symptoms, effective management of these side effects requires a multidisciplinary approach.
Quantitative sensory testing along with advanced neuroimaging represent more recent advances for use in diagnostic tools which have improved early detection. This enhancement makes prompt actions quite feasible.
Harmful effects of chemotherapy may be lessened with new approaches being developed. These strategies include pharmacological agents, lifestyle modifications, and also novel therapeutic approaches. However, research that still continues is important so that people fully understand all the mechanisms that underlie side effects induced by chemotherapy and develop targeted therapies that may prevent or else reverse nerve damage. Oncology along with neurology specialists collaborate toward optimizing patient outcomes plus ensure cancer survivors live better lives. A thorough patient-centered approach for managing neurological side effects will be vital to improve survival rates as well as the overall well-being of patients, as the field of cancer therapy keeps on developing.
Written by Lily Tumanyan MD
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
