
New Paper Alert: Neoadjuvant chemoradiotherapy followed by active surveillance versus standard surgery for oesophageal cancer (SANO trial):
The SANO trial investigated the efficacy of active surveillance compared to standard surgery in patients with esophagal cancer, who achieved a clinical complete response following neoadjuvant chemoradiotherapy. This multicenter, phase 3 trial demonstrated that active surveillance was non-inferior to standard surgery, with 2-year overall survival rates of 74% and 71%, respectively.
These findings suggest that, for select patients, active surveillance could be a viable alternative to immediate surgery, potentially preserving quality of life without compromising survival outcomes.
Titile: Neoadjuvant chemoradiotherapy followed by active surveillance versus standard surgery for esophageal cancer (SANO trial): a multicentre, stepped-wedge, cluster-randomised, non-inferiority, phase 3 trial
Authors:
Berend J van der Wilk, MD PhD, Ben M Eyck, MD PhD, Prof Bas P L Wijnhoven, MD PhD, Sjoerd M Lagarde, MD PhD, Prof Camiel Rosman, MD PhD, Bo J Noordman, MD PhD, Maria J Valkema, MD PhD, Tanya M Bisseling, MD PhD, Peter-Paul L O Coene, MD PhD, Marc J van Det, MD PhD, Jan Willem T Dekker, MD PhD, Jolanda M van Dieren, MD PhD, Michail Doukas, MD PhD, Stijn van Esser, MD PhD, W Edward Fiets, MD PhD, Henk H Hartgrink, MD PhD, Joos Heisterkamp, MD PhD, I Lisanne Holster, MD PhD, Bastiaan Klarenbeek, MD PhD, David van Klaveren, PhD, Eva Kouw, MD, Ewout A Kouwenhoven, MD PhD, Prof Misha D Luyer, MD PhD, Bianca Mostert, MD PhD, Grard A P Nieuwenhuijzen, MD PhD, Liekele E Oostenbrug, MD PhD, Prof Jean-Pierre Pierie, MD PhD, Johanna W van Sandick, MD PhD, Meindert N Sosef, MD PhD, Prof Manon C W Spaander, MD PhD, Roelf Valkema, MD PhD, Edwin S van der Zaag, MD PhD, Prof Ewout W Steyerberg, PhD, Prof J Jan B van Lanschot, MD PhD, SANO Study Group
Published in Lancet oncology, March 2025
Background
Esophageal cancer is a significant global health concern, ranking as the seventh most common cancer worldwide and the sixth leading cause of cancer-related mortality. The standard treatment for resectable esophageal cancer typically involves neoadjuvant chemoradiotherapy (nCRT) followed by surgical resection. However, this approach is associated with considerable morbidity and mortality. An alternative strategy, active surveillance (AS), proposes close monitoring after nCRT, reserving surgery only for cases showing residual or recurrent disease. The SANO trial aimed to evaluate whether active survival could offer non-inferior overall survival compared to standard surgery while potentially reducing treatment-related complications and improving quality of life.
Methods and Study Design of SANO trial
The SANO trial was a multicenter, stepped-wedge, cluster-randomized, non-inferiority, phase 3 trial conducted across multiple centers specializing in esophageal cancer treatment. Patients with resectable esophageal cancer who had completed nCRT and achieved a clinical complete response (cCR) were eligible for inclusion. Participants were randomized in clusters to either the AS group or the standard surgery group.
This multicenter, randomized, non-inferiority trial enrolled patients between Nov 8, 2017 – Jan 17, 2021. A total of 1,115 patients were screened, with 809 providing informed consent, leading to 776 final participants. Those achieving a clinical complete response (cCR) after nCRT were randomized into two groups:
- Active Surveillance (n=156)
- Standard Surgery (n=118)
An additional 35 patients from the preSANO trial were included in the standard surgery group.
Crossover occurred between groups, resulting in a modified intention-to-treat population of 198 AS and 111 standard surgery patients.
Patients were closely monitored with regular clinical response evaluations, and those in the AS group underwent surgery only if tumor regrowth occurred.
The primary endpoint was overall survival, with secondary endpoints including progression-free survival, health-related quality of life, and treatment-related morbidity and mortality.
Results of SANO trial
The findings from the SANO trial suggest that active surveillance is a safe and effective management strategy for patients with resectable esophageal cancer who achieve a clinical complete response after neoadjuvant chemoradiotherapy. This approach not only maintains overall survival rates but also reduces treatment-related complications and enhances quality of life. These results support the consideration of AS as a standard care option in this patient population, potentially leading to more personalized and less invasive treatment paradigms.
- Survival outcomes
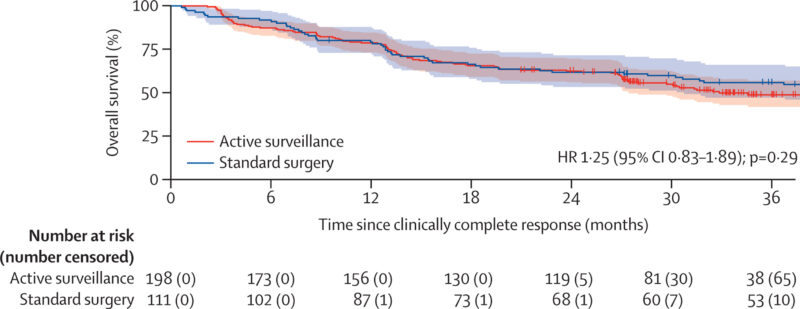
- Median survival: 43 months (AS) vs. 53 months (standard surgery)
- 2-year overall survival: 74% (95% CI 69–78) in AS vs 71% (95% CI 62–78) in standard surgery
- Intention-to-treat overall survival: 75% (95% CI 68–80) AS vs. 70% (95% CI 63–77) standard surgery
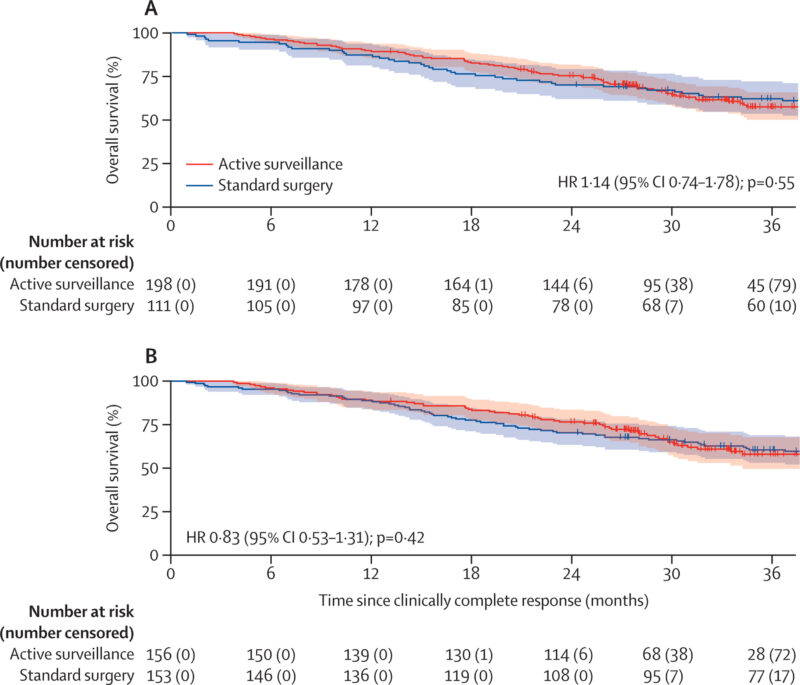
- Mortality rates: 50 deaths in the standard surgery group vs 79 deaths in the AS group (HR 1.14, 95% CI 0.74–1.78, p=0.55)
- Disease Progression & Recurrence:
- 33 patients (17%) in AS developed distant metastases without surgery
- 22 patients had metastases detected at 6-month follow-up
- 69 patients (35%) maintained a clinical complete response
- 96 patients (48%) developed isolated locoregional regrowth
- 90% of regrowth cases detected within 24 months post-nCRT
- 83 patients underwent salvage esophagectomy after a median of 5.9 months post-cCR
Key Takeaway Messages from SANO trial
- Non-Inferiority of Active Surveillance: The AS approach offers a viable alternative to immediate surgery for patients with resectable esophageal cancer who achieve cCR after nCRT, without compromising overall survival.
- Reduced Treatment-Related Complications: Patients managed with AS experienced fewer adverse events compared to those undergoing standard surgery, highlighting the potential of AS to minimize treatment-related morbidity.
- Improved Quality of Life: The AS group reported better health-related quality of life outcomes, suggesting that avoiding immediate surgery can preserve patient well-being.
- Selective Surgical Intervention: Implementing AS allows for surgery to be reserved for patients with residual or recurrent disease, reducing the number of unnecessary surgical procedures.
You can read the full article here
Written by Sona Karamyan, MD
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
