Enfortumab Vedotin, also known as Padcev, is a groundbreaking antibody-drug conjugate (ADC) designed to target Nectin-4. This innovative therapy combines a monoclonal antibody with a potent cytotoxic agent, monomethyl auristatin E (MMAE), disrupting cancer cell division and leading to cell death.
Which company produced Enfortumab Vedotin?
Enfortumab Vedotin was co-developed by Astellas Pharma and Seagen Inc. Astellas Pharma, a Japanese company formed in 2005, focuses on oncology, urology, immunology, and neurology. Seagen Inc., formerly Seattle Genetics, is a U.S. biotech firm specializing in antibody-drug conjugate (ADC) technology, which delivers targeted cancer treatment. In December 2023, Pfizer acquired Seagen for $43 billion, strengthening its oncology pipeline. Astellas and Seagen played a key role in advancing Padcev as a breakthrough therapy for urothelial carcinoma.
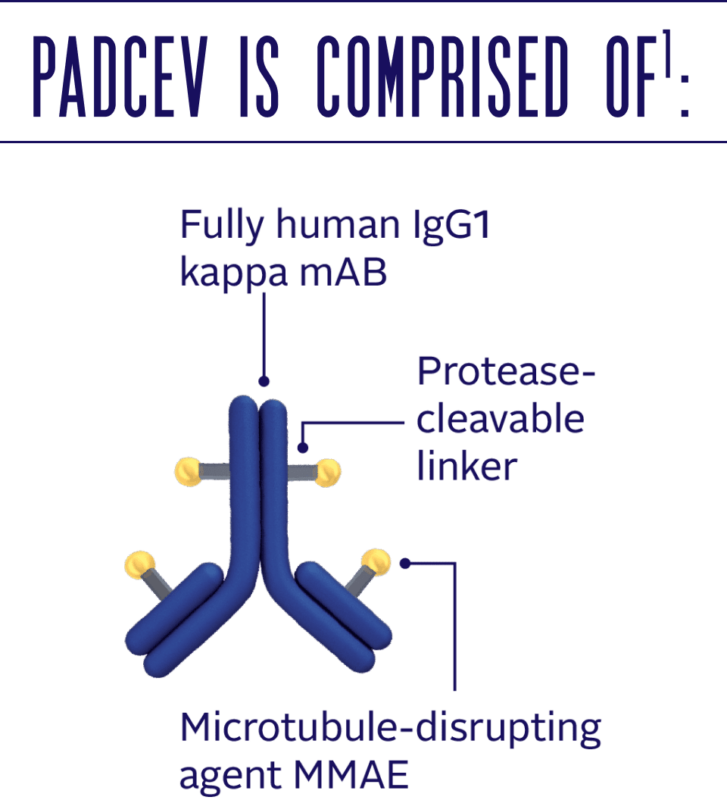
PADCEV® MECHANISM OF ACTION (MOA)
The story of Enfortumab vedotin’s approval
The journey of enfortumab vedotin began with its accelerated FDA approval on December 18, 2019, for patients with locally advanced or metastatic urothelial carcinoma who had previously been treated with a PD-1/L1 checkpoint inhibitor and platinum-based chemotherapy. The drug’s effectiveness was later confirmed in a large-scale clinical trial (EV-301), leading to full FDA approval on July 9, 2021.
Recognizing its potential beyond second-line therapy, researchers explored its use in earlier treatment settings. This led to a significant breakthrough on April 3, 2023, when the FDA approved enfortumab vedotin in combination with pembrolizumab as a first-line treatment for patients with advanced urothelial carcinoma who were ineligible for cisplatin-based chemotherapy.
How does Enfortumab Vedotin work?
Enfortumab vedotin works through a cutting-edge mechanism known as an antibody-drug conjugate (ADC), which combines the precision of targeted therapy with the power of chemotherapy. The process begins when the monoclonal antibody in enfortumab vedotin binds to Nectin-4, a protein found in high amounts on the surface of cancer cells, particularly in urothelial carcinoma.
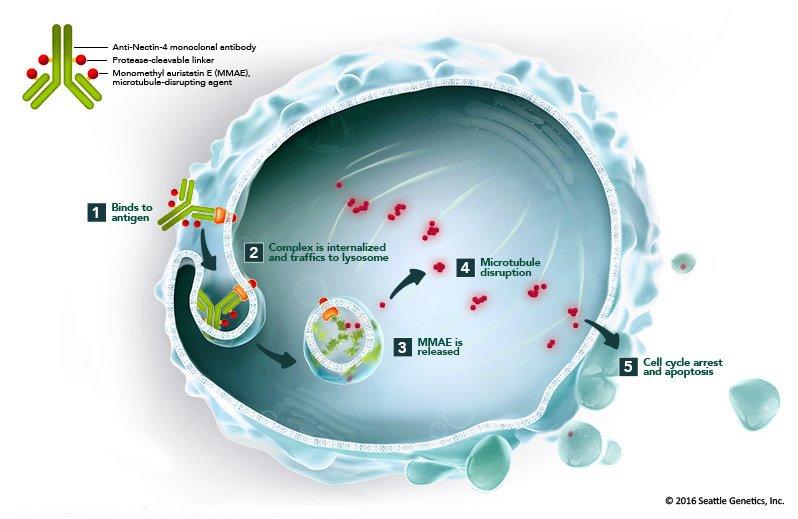
Once it attaches to the cancer cell, the drug is pulled inside. There, it releases its potent chemotherapy agent, monomethyl auristatin E (MMAE), which disrupts the cell’s microtubules, the structures responsible for cell division. With the cell unable to divide properly, it eventually dies. What makes enfortumab vedotin so effective is its selectivity. Targeting only the cancer cells that express Nectin-4 minimizes damage to healthy cells, reducing side effects often associated with traditional chemotherapy. This precise approach allows enfortumab vedotin to offer a powerful treatment option for patients with advanced bladder cancer, particularly those who have already exhausted other treatment options.
Understanding Antibody-Drug Conjugates: How They Target and Kill Cancer Cells?
What Cancers Is Enfortumab Vedotin Approved to Treat?
Enfortumab vedotin is approved for urothelial carcinoma, a cancer affecting the bladder and urinary tract. The FDA first approved it in December 2019 for advanced urothelial carcinoma in patients who had received prior PD-1/PD-L1 inhibitor and platinum-based chemotherapy. In April 2023, it was approved for first-line treatment in cisplatin-ineligible patients when combined with pembrolizumab, offering new options for those with advanced bladder cancer.
Learn more about Bladder Cancer, Symptoms ,Causes, Stages, Diagnosis and Treatment on OncoDaily.
What research is behind the approval?
The pivotal trial of Enfortumab Vedotin in urothelial carcinoma after platinum and anti–PD-1/L1 therapy was published in JCO on July 29, 2019. This phase II, single-arm study (EV-201) evaluated Enfortumab Vedotin in 125 patients with locally advanced or metastatic urothelial carcinoma previously treated with platinum and anti–PD-1/L1 therapy. The confirmed objective response rate was 44%, including 12% complete responses, with consistent efficacy across subgroups. The median response duration was 7.6 months. Common side effects included fatigue, peripheral neuropathy, alopecia, rash, and decreased appetite, with severe adverse events being rare. Enfortumab Vedotin demonstrated a meaningful response with a manageable safety profile in this heavily pretreated population.
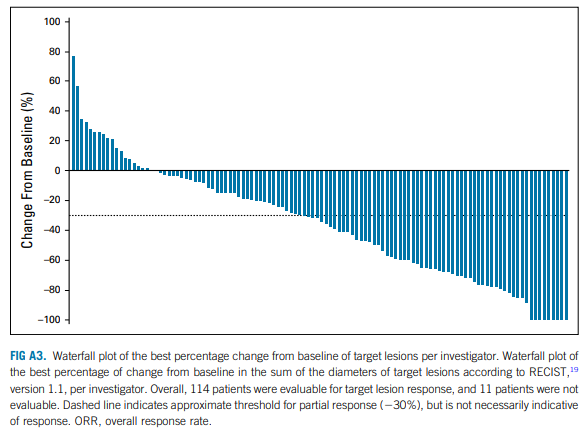
The phase 3 EV-301 trial, published in The New England Journal of Medicine on February 12, 2021, evaluated Enfortumab Vedotin in previously treated advanced urothelial carcinoma. In this global, open-label study, 608 patients who had progressed after platinum-based chemotherapy and PD-1/PD-L1 inhibitors were randomized to receive either Enfortumab Vedotin or standard chemotherapy. Enfortumab Vedotin significantly improved overall survival (12.9 vs. 9.0 months; HR 0.70, P = 0.001) and progression-free survival (5.6 vs. 3.7 months; HR 0.62, P < 0.001). Treatment-related adverse events were comparable between groups. These findings established Enfortumab Vedotin as a superior option over chemotherapy for this patient population.
The EV-103 Cohort K trial, published in JCO on June 27, 2023, studied Enfortumab Vedotin (EV) ± pembrolizumab (Pembro) as a first-line treatment for cisplatin-ineligible locally advanced/metastatic urothelial cancer. EV + Pembro showed a higher response rate (64.5% vs. 45.2%) and longer durability than EV alone. Side effects were manageable, with no new safety concerns. These findings support EV + Pembro as a promising first-line option.
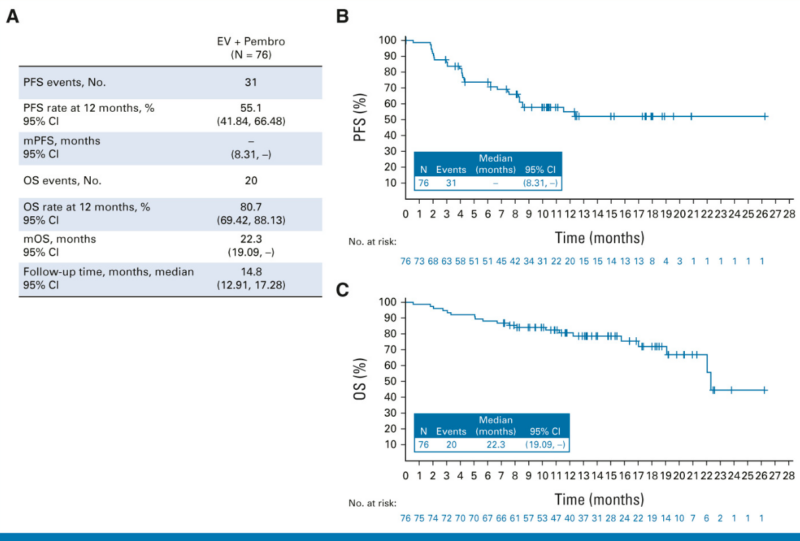
enfortumab vedotin-EV-103 trial for mts urothelial cancer
Enfortumab Vedotin Combinations and Treatment Outcomes
The EV-302 study published in The New England Journal of Medicine (March 2024) establishes this combination as a new standard of care. It showed that Enfortumab Vedotin plus pembrolizumab reduced the risk of disease progression or death by 55% and improved survival in urothelial cancer compared to chemotherapy. The combination led to higher response rates, with responses lasting over 12 months. It also showed longer progression-free (12.5 vs. 6.3 months) and overall survival (31.5 vs. 16.1 months).
The combination was well-tolerated, with fewer grade 3 or higher adverse events (55.9% vs. 69.5% for chemotherapy). Common side effects included skin reactions, neuropathy, and hyperglycemia. These studies support EV plus pembrolizumab as an effective and manageable treatment for advanced UC, offering better survival and response rates than chemotherapy. In the EV-103 Cohort K study, the combination achieved a 64.5% response rate in cisplatin-ineligible patients, with 65.4% of responders maintaining their responses for over a year, suggesting its potential as a first-line treatment for this group.
Enfortumab Vedotin side effects and its management
Enfortumab Vedotin is an antibody-drug conjugate used to treat urothelial cancer. While effective, it can cause various side effects that require careful management.
Common Side Effects
Patients on Enfortumab Vedotin often experience fatigue, with many feeling tired or weak. Peripheral neuropathy is another common side effect, leading to tingling, numbness, or pain in the hands and feet. A decreased appetite can lead to unintended weight loss, while skin reactions such as rashes, itching, redness, or peeling skin may occur. Hair loss, or alopecia, is frequently observed, and gastrointestinal issues like nausea, vomiting, diarrhea, and constipation are common. Eye problems, including dry eyes, blurred vision, or eye redness, can also develop, as well as altered taste and weight loss due to appetite reduction.
Serious Side Effects
In rarer cases, Enfortumab Vedotin can cause severe skin reactions, such as blistering and peeling, particularly during the first treatment cycle. These issues require immediate medical attention. Lung problems, including shortness of breath, cough, or fever, can signal serious complications. Eye issues, including vision changes and eye pain, also need prompt medical evaluation.
Management Strategies
Regular monitoring by healthcare providers is essential to effectively manage these side effects. Medications may be prescribed to alleviate symptoms like pain or nausea, and dose adjustments can help reduce the severity of side effects. Supportive care, such as working with nutritionists and counselors, is also helpful in maintaining a patient’s quality of life. Patients must communicate openly with their healthcare team to address any side effects early and implement personalized management strategies.
What is the Recommended Dosage of Enfortumab Vedotin?
Enfortumab Vedotin is recommended at a dosage of 1.25 mg/kg of body weight, up to a maximum of 125 mg, for patients weighing 100 kg or more. It is administered intravenously over 30 minutes on days 1, 8, and 15 of a 28-day cycle when used alone. If combined with pembrolizumab, it is given on days 1 and 8 of a 21-day cycle, with Padcev administered before pembrolizumab. Treatment continues until disease progression or intolerable side effects occur, and dosage adjustments may be necessary based on patient response.
How is Enfortumab Vedotin administered?
Enfortumab Vedotin (Padcev) is administered as an intravenous (IV) infusion. It should be given over 30 minutes. It must not be administered as an IV push or bolus. When used in combination with pembrolizumab, Enfortumab Vedotin should be given first, followed by pembrolizumab if both are administered on the same day.
For storage, do not freeze or shake the medication. Unused vials should be kept in the original carton and stored in the refrigerator at 2-8ºC (36-46ºF). Reconstituted vials, which lack preservatives, should be refrigerated and used within 4 hours. Diluted solutions can be refrigerated for up to 8 hours if not administered immediately. The medication must be reconstituted and diluted before infusion.
Learn more about Immunotherapy for Urothelial Cancer: Types, Success Rate, Side Effects on OncoDaily.
What to Avoid During Enfortumab Vedotin Treatment?
During Enfortumab Vedotin treatment, there are several precautions to keep in mind. First, it’s crucial not to miss any doses, as sticking to the prescribed schedule is essential for the treatment to be effective. Since Enfortumab Vedotin can weaken the immune system, patients should avoid exposure to infections and practice good hygiene. Grapefruit and grapefruit juice should also be avoided, as they can interact with the medication and affect how the body processes it, potentially reducing its effectiveness. Live vaccines are not recommended during treatment, as the drug can interfere with the body’s immune response. Pregnant women should avoid Enfortumab Vedotin, as it can harm an unborn baby.
Both men and women should use effective contraception during and after treatment. Finally, patients should be cautious about sun exposure. Since Enfortumab Vedotin can cause skin reactions, it’s important to protect the skin from the sun and use sunscreen. Certain medications should be avoided or used with caution while on Enfortumab Vedotin. CYP3A4 inhibitors (e.g., ketoconazole) can increase drug toxicity, while CYP3A4 inducers (e.g., rifampin) can reduce its effectiveness. Additionally, P-glycoprotein (P-gp) inhibitors and inducers can alter drug levels. Patients with pre-existing neuropathy, diabetes, or liver disease should inform their oncologist before starting treatment. Activities that increase the risk of cuts or bruises, such as contact sports, should also be avoided due to the potential for increased bleeding risk.
Enfortumab Vedotin effectiveness over time
Enfortumab Vedotin has demonstrated sustained effectiveness over time in treating locally advanced or metastatic urothelial carcinoma. In clinical trials, the drug has shown durable responses in patients, with some experiencing long-lasting effects. In the EV-302 trial, the combination of Enfortumab Vedotin and pembrolizumab led to 12.5 months of progression-free survival (PFS) and 31.5 months of overall survival (OS), significantly better than chemotherapy. In the EV-103 Cohort K study, the combination had a 64.5% overall response rate, with 65.4% of responders maintaining their responses for over a year.
These results demonstrate Enfortumab Vedotin’s long-term effectiveness, especially when combined with pembrolizumab, improving survival and response durations in advanced urothelial cancer.
The EV-202 Phase II study tested Enfortumab Vedotin (EV) in patients with unresectable recurrent/metastatic head and neck cancer (HNC) who had received prior treatments. The study showed a 23.9% objective response rate and a 56.5% disease control rate. The median duration of response was 9.4 months, with 3.9 months progression-free survival and 6.0 months overall survival. Common side effects included alopecia, fatigue, and neuropathy. These results support further investigation of EV for advanced HNC.
Learn more about Targeted Therapy vs Immunotherapy: Which Is Right for You, Key Differences & Similarities on OncoDaily.
Ongoing trials with Enfortumab Vedotin
Ongoing clinical trials are exploring the potential of Enfortumab Vedotin (PADCEV) beyond urothelial carcinoma, targeting multiple solid tumors. A Phase II study (NCT06553885) is evaluating its efficacy in advanced or metastatic colorectal cancer (CRC) and hepatocellular carcinoma (HCC) in patients who have progressed after standard systemic therapies. Another Phase II trial (NCT05915351) is investigating its role in previously treated pancreatic adenocarcinoma, aiming to determine its safety and effectiveness in this aggressive cancer type. These studies focus on tumors expressing Nectin-4, the primary target of Enfortumab Vedotin, potentially expanding its use in difficult-to-treat malignancies.
Dr. Mohammad Jad Moussa on Small Cell Bladder Cancer: Relapse Risks & pCR Impact | OncoDaily
Written by Mariam Khachatryan, MD
FAQ
What is Enfortumab Vedotin used for?
Enfortumab Vedotin is an FDA-approved drug used to treat locally advanced or metastatic urothelial carcinoma (UC), a type of bladder cancer. It is also being studied for potential use in head and neck cancer (HNC), colorectal cancer (CRC), and hepatocellular carcinoma (HCC).
How does Enfortumab Vedotin work?
Enfortumab Vedotin is an antibody-drug conjugate that targets nectin-4, a protein found on the surface of cancer cells. The drug delivers a chemotherapy agent directly to the cancer cells, improving its ability to kill tumors while minimizing damage to healthy tissues
What are the common side effects of Enfortumab Vedotin?
Common side effects of Enfortumab Vedotin include alopecia (hair loss), fatigue, peripheral neuropathy (nerve damage), skin reactions, and hyperglycemia (high blood sugar). Most side effects are manageable, but patients should report any concerning symptoms to their doctor.
How is Enfortumab Vedotin administered?
Enfortumab Vedotin is administered as an intravenous (IV) infusion. The typical dosage is 1.25 mg/kg every 3 weeks, with the treatment schedule dependent on the patient’s condition and response
Are there any contraindications for using Enfortumab Vedotin?
Enfortumab Vedotin should not be used in patients with a known hypersensitivity to the drug or its components. It is important to inform your doctor of any allergies, especially to monoclonal antibodies.
How long does it take for Enfortumab Vedotin to work?
The time for Enfortumab Vedotin to show noticeable effects varies between patients. However, the response duration can be extended, with some patients experiencing ongoing responses for up to a year.
Can Enfortumab Vedotin be combined with other cancer treatments?
Yes, Enfortumab Vedotin is often used in combination with other cancer therapies, such as pembrolizumab, an immune checkpoint inhibitor, to enhance its effectiveness in treating advanced cancers, particularly urothelial carcinoma.
What should I do if I miss a dose of Enfortumab Vedotin?
If you miss a dose of Enfortumab Vedotin, contact your doctor immediately. They will provide instructions on rescheduling your treatment to ensure you stay on track with your therapy.