New Paper Alert: Botensilimab and Balstilimab in Relapsed/Refractory Metastatic Sarcomas
In this Summary, we discuss the new paper published in JCO on Botensilimab and Balstilimab, which Show Promising Results in Sarcoma Treatment.
Title: Botensilimab (Fc-enhanced anti–cytotoxic lymphocyte-association protein-4 antibody) Plus Balstilimab (anti–PD-1 antibody) in Patients With Relapsed/Refractory Metastatic Sarcomas.
Published in Journal of Clinical Oncology, on January 27, 2025
Authors: Breelyn A. Wilky, MD; Gary K. Schwartz, MD; Michael S. Gordon, MD; Anthony B. El-Khoueiry, MD; Andrea J. Bullock, MD; Brian Henick, MD; Mark Agulnik, MD; Arun Singh, MD; Daruka Mahadevan, MD, PhD; Justin Stebbing, MD, PhD; Chloe Delepine, PhD; Dhan Chand, PhD; Manushak Avagyan, MD; Wei Wu, MS; Benny Johnson, DO; Joseph E. Grossman, MD; Steven O’Day, MD; Jonathan C. Trent, MD, PhD; Robin L. Jones, MD, MRCP; Apostolia M. Tsimberidou, MD, PhD
Key Takeaways
- Novel combination immunotherapy shows promising efficacy in traditionally immunotherapy-resistant sarcomas
- Overall response rate of 19.2% across multiple sarcoma subtypes
- Manageable safety profile with no grade 4-5 treatment-related adverse events
- Particularly effective in angiosarcoma with 27.8% response rate
- Durable responses observed with median duration of 21.7 months
Background
Sarcomas represent a heterogeneous group of rare cancers with poor prognosis in the metastatic setting, with 5-year survival rates of approximately 16%. Traditional immunotherapy approaches have shown limited efficacy due to:
- Low tumor mutational burden
- Suppressive microenvironments
- Dysregulated antigen presentation
- Low expression of immune-related genes
Previous attempts at immunotherapy in sarcomas have yielded modest results, with PD-1 monotherapy showing response rates below 10% in common adult subtypes such as leiomyosarcoma and dedifferentiated liposarcoma. One notable exception has been cutaneous angiosarcoma, particularly those arising in the face and scalp, which exhibit an ultraviolet genetic signature similar to melanoma and have shown more encouraging responses to immunotherapy. This heterogeneity in response patterns highlights the need for more effective and broadly applicable immunotherapeutic strategies for sarcoma treatment.
The Study Drugs
Botensilimab (BOT) represents a novel approach in immunotherapy as an Fc-enhanced anti-CTLA-4 antibody. Its engineered IgG1 region provides superior binding to activating Fc-gamma receptors compared to conventional anti-CTLA-4 antibodies like ipilimumab, leading to enhanced engagement between T cells and antigen-presenting cells. This unique design promotes improved T-cell priming, activation, and memory formation. Its companion drug, balstilimab (BAL), is a fully humanized anti-PD-1 antibody that demonstrates activity comparable to other approved PD-1 inhibitors. The combination of these agents is designed to provide complementary immune activation, with BOT’s enhanced Fc-effector functions potentially creating a more favorable environment for BAL’s checkpoint inhibition.
Study Design and Methods
- Phase I trial evaluating botensilimab (BOT, anti-CTLA-4) plus balstilimab (BAL, anti-PD-1)
- 64 patients enrolled and treated between October 2020 and January 2024
- Dosing:
- BOT: 1 mg/kg or 2 mg/kg IV every 6 weeks
- BAL: 3 mg/kg IV every 2 weeks
- Primary endpoint: Safety and toxicity
- Secondary endpoints: ORR, DOR, DCR, PFS
- Median follow-up: 9.1 months
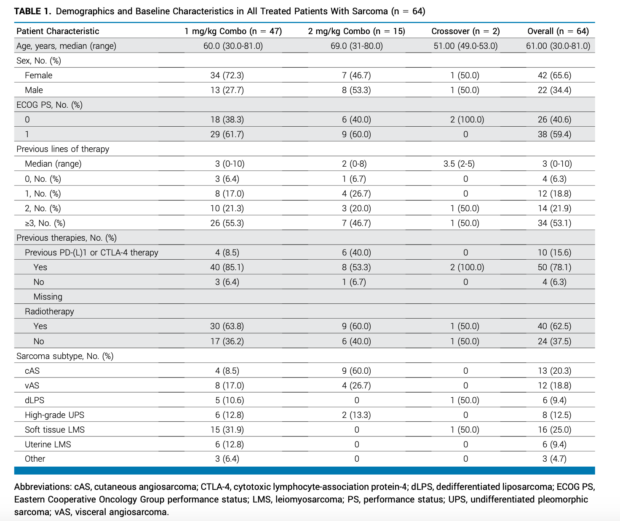
Patient Characteristics
- Median age: 61 years (range 30-81)
- Female: 65.6%
- ECOG PS: 0 (40.6%) or 1 (59.4%)
- Median prior lines of therapy: 3 (range 0-10)
- Key sarcoma subtypes:
- Angiosarcoma: 39.1% (25/64)
- Cutaneous: 20.3%
- Visceral: 18.8%
- Leiomyosarcoma: 34.4% (22/64)
- Soft tissue: 25.0%
- Uterine: 9.4%
- Angiosarcoma: 39.1% (25/64)
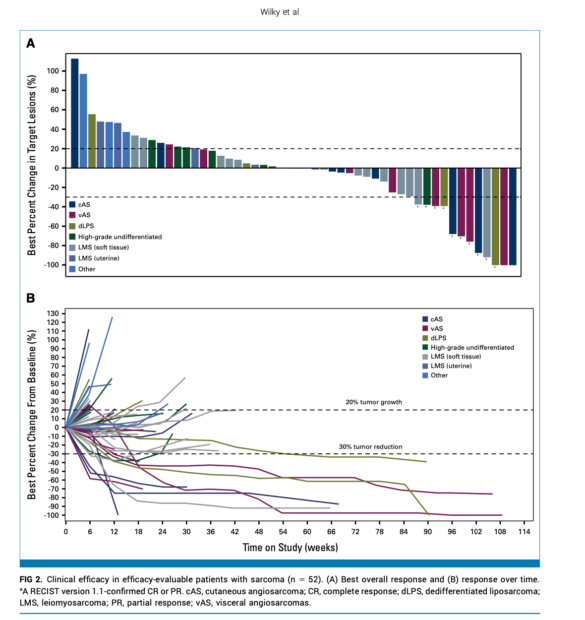
Efficacy Results
- Overall Response Rate (RECIST 1.1): 19.2% (95% CI: 9.6-32.5)
- Disease Control Rate: 65.4% (95% CI: 50.9-78.0)
- Clinical Benefit Rate: 32.7% (95% CI: 20.3-47.1)
- Median PFS: 4.4 months (95% CI: 2.8-6.1)
- 6-month PFS rate: 36% (95% CI: 21.9-50.2)
- Median Duration of Response: 21.7 months (95% CI: 1.9-NR)
- 12-month OS rate: 69% (95% CI: 52.3-80.8)
Subtype-Specific Results
Angiosarcoma (n=18):
- ORR: 27.8%
- DCR: 77.8%
- Median PFS: 4.7 months
- Response rates:
- Cutaneous: 22.2%
- Visceral: 33.3%
Soft Tissue Leiomyosarcoma (n=15):
- ORR: 13.3%
- DCR: 73.3%
Safety Profile
- Treatment-related adverse events (TRAEs): 82.8% of patients
- Grade 3 TRAEs: 17.2%
- No grade 4-5 TRAEs reported
- Most common TRAE: Diarrhea/colitis (35.9%, grade 3: 6.3%)
- Other common TRAEs:
- Fatigue: 26.6%
- Pyrexia: 21.9%
- Chills: 17.2%
- Rash: 17.2%
Biomarker Analysis
- Lower peripheral IL-6 levels significantly associated with improved OS
- Trend toward improved survival with PD-L1 CPS ≥1
- Most tumors exhibited low TMB (<10 mut/Mb)
- FCGR3A status did not significantly affect outcomes
Clinical Implications
This combination therapy shows promising activity in traditionally immunotherapy-resistant sarcomas, with particularly encouraging results in angiosarcoma, including visceral subtypes. The safety profile appears manageable with appropriate monitoring and intervention strategies. The durable responses observed suggest potential long-term benefits for responding patients.
Future Directions of Botensilimab and Balstilimab in Sarcoma
Further investigation is warranted to:
- Confirm efficacy in larger patient populations
- Identify predictive biomarkers
- Optimize patient selection
- Evaluate combination strategies
- Study earlier lines of therapy
You can Read the Full article in the Journal of Clinical Oncology

Summary by Amalya, Sargsyan, MD
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023