A recent paper by Toshinari Yamashita et al. was published in the Journal of Clinical Oncology:
Authors: Toshinari Yamashita, Shigehira Saji, Toshimi Takano, Yoichi Naito, Michiko Tsuneizumi, Akiyo Yoshimura, Masato Takahashi, Junji Tsurutani, Tsuguo Iwatani, Masahiro Kitada, Hiroshi Tada, Natsuko Mori, Toru Higuchi, Tsutomu Iwasa, Kazuhiro Araki, Kei Koizumi, Hiroki Hasegawa, Yohei Uchida, Satoshi Morita and Norikazu Masuda.
Purpose:
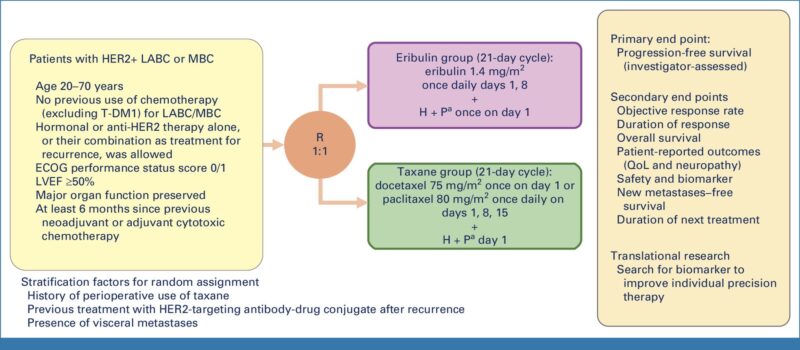
The study aimed to evaluate the noninferiority of eribulin compared to taxane when combined with trastuzumab and pertuzumab (HP) as first-line treatment for recurrent or metastatic HER2-positive breast cancer.
Methods:
In the phase III EMERALD trial (NCT03264547), 446 patients were randomly assigned to receive either eribulin (1.4 mg/m² on days 1 and 8) or a taxane (docetaxel or paclitaxel) in 21-day cycles, both alongside HP. The primary endpoint was progression-free survival (PFS), with secondary endpoints including overall survival (OS), quality of life (QoL), and safety.
Results:
The median PFS was 14.0 months for the eribulin group and 12.9 months for the taxane group, confirming noninferiority (HR, 0.95; 95% CI, 0.76 to 1.19). The median OS was not reached for eribulin but was 65.3 months for taxane. Adverse events were similar between groups, although neutropenia was more common with eribulin.
Conclusion:
Eribulin combined with HP is a viable first-line treatment option for locally advanced/metastatic HER2-positive breast cancer, demonstrating noninferiority to taxanes in terms of PFS and comparable safety profiles.
Doctors and healthcare influencers have shared their insights about the article:
Elisa Agostinetto, Medical Research Fellow at the University Hospital of Brussels:
“Trastuzumab-pertuzumab-taxane is the SoC 1st line for metastatic HER2+ breast cancer; yet, taxane may induce neurotoxicity. The EMERALD trial now out in JCO investigated the role of eribulin instead of a taxane in this setting.”
Elisabetta Bonzano, Radiation Oncologist at IRCCS San Matteo Polyclinic Foundation:
“Trastuzumab-Pertuzumab Plus Eribulin or Taxane as First-Line Chemotherapy for Human Epidermal Growth Factor 2–Positive Locally Advanced/Metastatic Breast Cancer: The Randomized Noninferiority Phase III EMERALD Trial.
The results suggested that eribulin and HP is an option for first-line treatment of locally advanced/metastatic HER2+ BC.”
More posts featuring Elisa Agostinetto and Elisabetta Bonzano.