Al-Ola A Abdallah, Plasma Cell Disorder Program Director, Division of HMCT/University of Kansas Medical Center, shared an article by Yael C. Cohen, et al, on X:
“Now out RedirectTT–1 study!
New questions, that I will answer at the end of the thread.
- Is this clinically impactful in the real world?
- When will you use this approved combination?
- Is it worth getting published?
Phase 1b-2 RedirecTT–1 study, which investigated the combination of two bispecific antibodies, talquetamab (targeting GPRC5D) and teclistamab (targeting BCMA), in patients with RRMM who were triple-class exposed.
The study hypothesized that dual targeting of BCMA and GPRC5D could enhance treatment potency, overcome antigen escape, and improve response durability!
Both Teclistamab and Talquetamab proved their efficacy as a single agent in prior trials.
Study Design: Phase 1b-2, open-label, non-randomized, multicenter study
- Five dose levels were tested.
- The recommended phase 2 regimen was talquetamab 0.8 mg/kg plus teclistamab 3.0 mg/kg every two weeks.
- Patients were required to have triple-class exposed RRMM and ECOG of zero or one.
- Patients could transition from bi-weekly to monthly dosing after a partial response or better after cycle 4.
94 patients received treatment (44 with the recommended phase 2 regimen).
- The Median follow-up was 20.3 months overall and 18.2 months for the recommended phase 2 regimen.
- The median age was 64.5 years.
- Median of 4 prior LOT.
- All patients had triple-class exposure; 65 percent had penta-drug exposure.
- 86 percent triple-class refractory.
- 36 percent had EMD. (Small Number).
- 41 percent had a high-risk cytogenetic.
Efficacy:
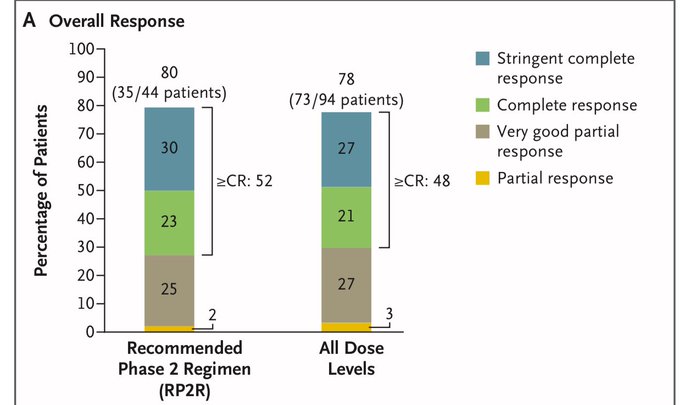
- Recommended Phase 2 Regimen: ORR (80 percent), VGPR=>77 percent, CR>=52 percent.
- The median time to respond was 1.4 months.
- Across all dose levels: 78 percent ORR, 74 percent VGPR>=, 48 percent CR>=.
- The median time to respond was 1.8 months.
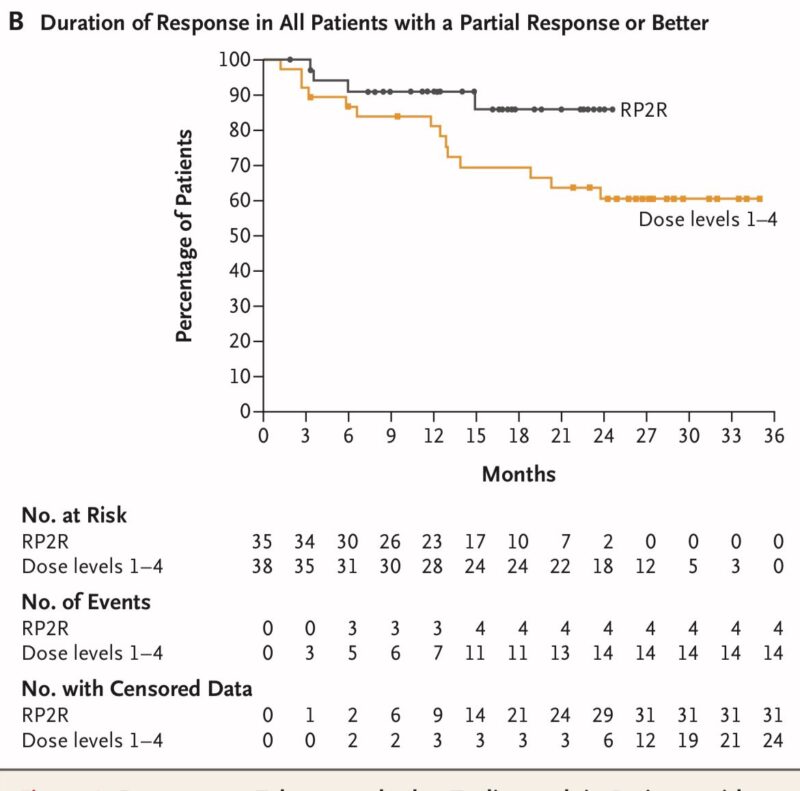
- DOR: Continuing response at 18 months was 86 percent (recommended phase 2 regimen) and 77 percent (all dose levels).
Content Efficacy:
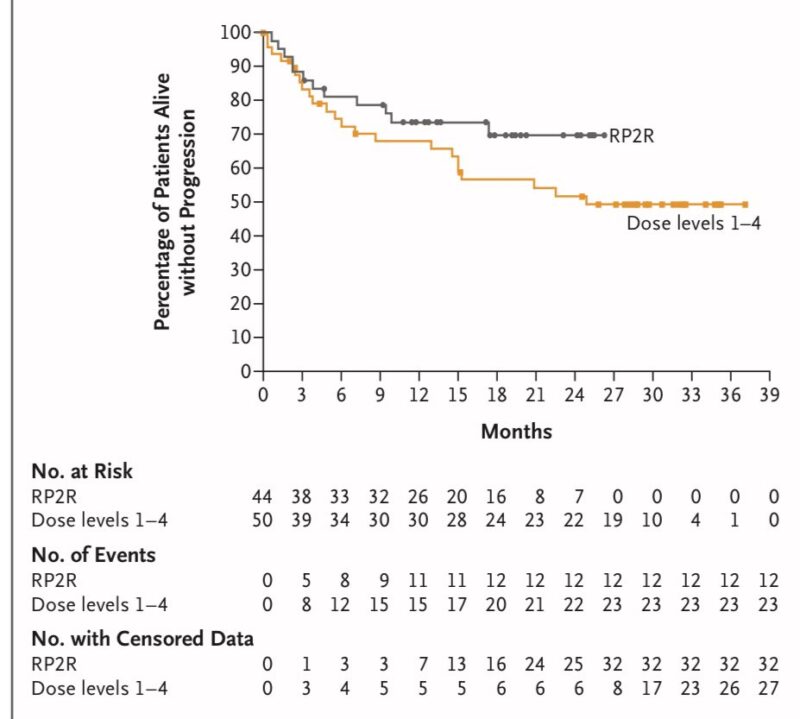
- 82 percent of patients with EMD on the recommended dose continued response at 18 months. 52 percent of patients with EMD across all dose levels at 18 months.
- PFS at 18 months: 70 percent for the recommended phase 2 regimen and 62 percent for all dose levels.
- Responses were maintained or deepened when transitioning from bi-weekly to monthly dosing.
Safety:
- 3 patients experienced dose-limiting toxic effects, including 1 grade 4 thrombocytopenia at the recommended phase 2 regimen.
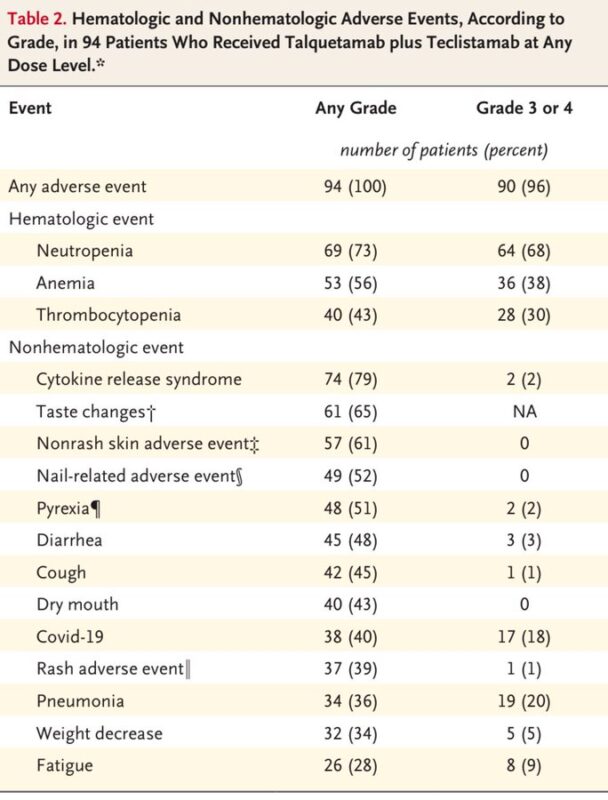
- Most common adverse events: CRS, neutropenia, taste changes, and non-rash skin events.
- Grade 3/4 adverse events occurred in 96 percent of patients, most commonly hematologic (80 percent).
- Grade 3/4 infections occurred in 64 percent of patients; higher than seen with monotherapy. – Infections were the primary cause of cycle delays/dose modifications (68 percent) and discontinuations (5 percent).
Content Safety:
- TRM due to AEs was 15 percent; caused by infection 11 grade 5.
- The incidence of grade 3 or 4 infections with talquetamab plus teclistamab was higher than has been observed with either therapy alone.
- CRS was common (79 percent), mostly grade 1 or 2.
- 2 patients with grade 3 CRS and 0 with grade 4 or 5.
- ICANS was rare (3 patients), reversible, and grade 1-3.
- Taste changes (65 percent), skin-related adverse events (39 percent rash, 61 percent non-rash), and nail adverse events (52 percent) were also frequent.
Summary:
- Effective treatment
- AEs especially the infection rate is horrible and TRM!
- Still AEs from Talquetamab that impact QOL including taste changes are an issue!
- I will not use this combination therapy unless there are no options for young, fit patients with high risk and multiple relapses. I will not recommend using it until safety is better understood.
- I’m not sure why NEJM accepted this paper, as it didn’t provide any new insights for our practice.”
Talquetamab plus Teclistamab in Relapsed or Refractory Multiple Myeloma

Authors: Yael C. Cohen, Hila Magen, Moshe Gatt, Michael Sebag, Kihyun Kim, Chang-Ki Min, Enrique M. Ocio, Sung-Soo Yoon, Michael P. Chu, Paula Rodríguez-Otero, Irit Avivi, Natalia A. Quijano Cardé, Ashwini Kumar, Maria Krevvata, Michelle R. Peterson, Lilla Di Scala, Emma Scott, Brandi Hilder, Jill Vanak, Arnob Banerjee, Albert Oriol, Daniel Morillo, María-Victoria Mateos.
More posts featuring Al-Ola A Abdallah.