Marco Donia shared on LinkedIn:
“Three-Year Overall Survival with Nivolumab + Relatlimab (rela-nivo) in Advanced Melanoma
Authors: Hussein A. Tawbi, F. Stephen Hodi, Evan J. Lipson, Dirk Schadendorf, Paolo A. Ascierto, Luis Matamala, Erika Castillo Gutiérrez, Piotr Rutkowski, Helen Gogas, Christopher D. Lao, Juliana Janoski De Menezes, Stéphane Dalle, Ana Maria Arance, Jean-Jacques Grob, Barbara Ratto, Saima Rodriguez, Antonella Mazzei, Sonia Dolfi, and Georgina V. Long

(Hussein Tawbi et al., J Clin Oncol, Dec 2024; Free Access)
Key Findings (median follow-up 33.8 months of RELATIVITY-047):
Progression-Free Survival (PFS) and Overall Survival (OS):
Median PFS: 10.2 months (rela-nivo) vs. 4.6 months (nivo alone).
Hazard Ratio (HR): 0.79 (95% CI, 0.66–0.95).
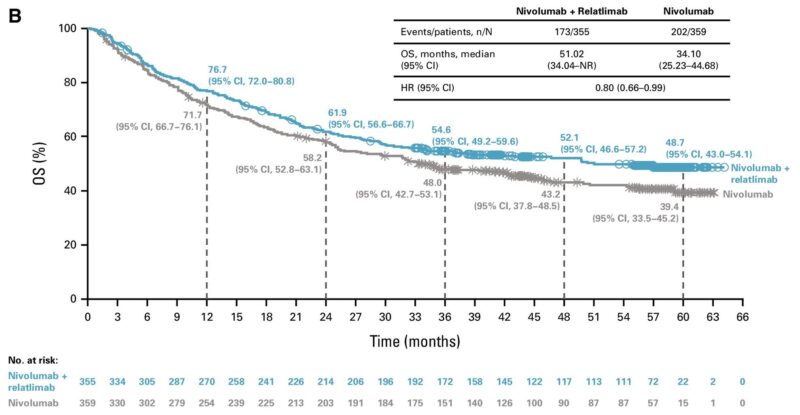
Median OS: 51.0 months (rela-nivo) vs. 34.1 months (nivo alone).
HR: 0.80 (95% CI, 0.66–0.99).
Safety Profile:
Grade 3-4 Treatment-Related Adverse Events (TRAEs):
Rela-nivo: 22.0%.
Nivo alone: 12.0%.
For comparison, Ipi-Nivo Grade 3-4 TRAEs reported at >50% across studies in melanoma
Subgroup Analysis*:
PD-L1 <1%: Rela-nivo showed a clear benefit in PFS (HR: 0.68, 95% CI: 0.54–0.86) and favorable OS (HR: 0.83, 95% CI: 0.64–1.07).
PD-L1 ≥1%: Despite no improvement in PFS (HR: 0.98, 95% CI: 0.73–1.32), OS favored rela-nivo with an HR of 0.78 (95% CI, 0.56–1.08).
Clinical Implications:
This new analysis reaffirms nivo+rela as a safe and effective treatment option for patients with treatment-naive, advanced melanoma without CNS metastases.
Favorable OS compared to Nivo alone regardless PD-L1 status and across most subgroups
Nivo-rela or Ipi/Nivo? An indirect analysis
Commentary:
The observed favorable OS in the PD-L1 ≥1% subgroup is reminiscent of the 2010 ipilimumab (+/- gp100 vaccine) vs gp100 vaccine trial, Improved Survival with Ipilimumab in Patients with Metastatic Melanoma
where long-term immune modulation led to OS gains despite nearly identical median PFS. This suggests potential long-term immune modulation mechanisms.
Nivo-Rela was approved in 2022 by European Medicines Agency for patients with PD-L1 <1% only based on PFS advantage in this subgroup after median 19 months follow-up, more details and extensively At this earlier follow-up, favorable OS outcomes were less clear, especially for PD-L1 ≥1% (HR: 0.84, 95% CI: 0.57–1.24), See NEJM Evidence and discussion.”
Marco Donia is a Senior Consultant, Clinician-Scientist and Junior Research Group Leader (TIL group) at the Department of Oncology and Center for Cancer Immune Therapy, University of Copenhagen Herlev Hospital, Denmark. He serves as an Associate Professor in Clinical Oncology at the University of Copenhagen.
Donia is also a clinical oncologist treating patients with cancer immunotherapy. His research group is currently investigating new immune-regulatory circuits in PD-1/PD-L1 resistant tumors.