
Funding Success: March Biosciences Closes Oversubscribed $28.4 Million Series A Financing
March Biosciences (March Bio), an emerging clinical-stage biotechnology company focused on addressing challenging cancers and other diseases, has successfully closed a $28.4 million oversubscribed Series A financing round.
The financing was led by Mission BioCapital and 4BIO Capital, with participation from KdT Ventures, Alexandria Venture Investments, Volnay Therapeutics, Modi Ventures, Mansueto Investments, and existing investors including TMC Venture Fund, Cancer Focus Fund, Small Ventures, and Portal Innovations. To date, March Bio has raised over $51 million in total funding.

March Biosciences, based in Houston, is a biotechnology company focused on addressing challenging cancers that do not respond to current immunotherapies. Originating from the Center for Cell and Gene Therapy at Baylor College of Medicine, Houston Methodist Hospital, and Texas Children’s Hospital, the company is advancing its lead asset, MB-105, a CD5-targeted CAR-T cell therapy.
Currently in Phase 1 trials for refractory T-cell lymphoma and leukemia, MB-105 has shown promising efficacy and safety signals. A Phase 2 trial is anticipated to begin early next year. To date, March Biosciences has raised over $50 million, supported by funding from the Cancer Prevention and Research Institute of Texas (CPRIT) and the NIH SBIR program.
“This oversubscribed financing enables us to advance our first-in-class CAR-T therapy, MB-105, into a Phase 2 trial for T-cell lymphoma – an indication with an exceptionally poor prognosis and few treatment options.
With the support and confidence of our investors, we are not only advancing our lead program but also expanding our pipeline, underscoring our commitment to delivering best-in-class therapies to patients that can change the treatment paradigm for these challenging cancers.” – said Sarah Hein, Co-Founder and Chief Executive Officer of March Biosciences.
4BIO Capital is an international venture capital firm that invests in advanced therapies, including genomic medicines and emerging technologies. The firm aims to support early-stage companies developing treatments for areas with high unmet medical needs, ensuring access to potentially curative therapies for patients.
“For far too long, T-cell cancers have been an innovation desert with patients facing a dismal prognosis. March Bio’s innovative autologous CAR-T approach brings patients new hope.
MB-105 is specifically engineered for relapsed and refractory CD5 positive T-cell lymphomas and I am delighted that this targeted approach combined with the incredible team led by Sarah is moving rapidly into Phase 2 to bring this exciting new treatment to patients.
We are honored to be a co-lead investor in March Bio and to help support the company as it continues in its mission to bring transformative therapies to those in urgent need.” – said Owen Smith, Partner at 4BIO Capital
The team at 4BIO includes leading scientists and experienced life science investors who have published extensively in prestigious journals. The firm seeks high-quality opportunities in cell and gene therapy, RNA-based therapy, targeted therapies, and the microbiome.
Mission BioCapital focuses on early-stage investments in life sciences companies. With offices in leading life sciences hubs like Cambridge, MA, and San Francisco, the firm provides portfolio companies with shared lab space, capital investment, and access to strategic partners. Mission BioCapital is committed to helping entrepreneurial scientists build successful companies from inception to exit.
“The team at March Biosciences is leveraging powerful science and promising clinical data to tackle cancers with significant unmet need. We’re excited to support their journey and believe their focused approach with MB-105 could lead to significant breakthroughs in the CAR-T space.” – said Cassidy Blundell, Partner at Mission BioCapital.
Since its inception as a spinout from the Center for Cell and Gene Therapy (Baylor College of Medicine, Houston Methodist Hospital, Texas Children’s Hospital), March Bio has been advancing its innovative autologous chimeric antigen receptor T-cell (CAR-T) therapy, MB-105.
This therapy is in development for treating relapsed and refractory CD5 positive T-cell lymphoma. MB-105 is engineered to overcome significant challenges related to T-cell targeting while maintaining high potency against CD5 positive tumor cells. Early results from a Phase 1 clinical trial at Baylor College of Medicine have shown a favorable safety profile and durable remissions in patients with relapsed T-cell lymphoma.
The company plans to initiate a Phase 2 clinical trial in early 2025, with proceeds from the financing allocated to support this development.
March Biosciences and MB-105
MB-105 is a CD5 CAR T-cell technology invented at Baylor College of Medicine.
MB-105 is a CAR-T cell therapy that selectively targets CD5, an antigen commonly expressed in both normal and malignant T-cells. This innovative therapy addresses significant challenges associated with CAR technologies in T-cell malignancies by leveraging proprietary manufacturing processes and an optimized CAR design.
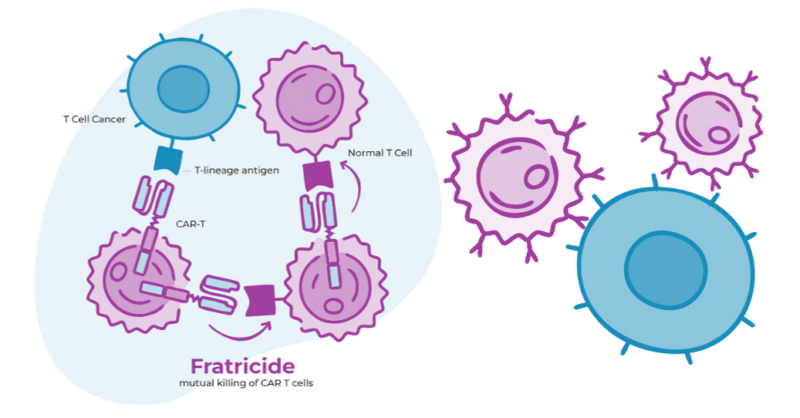
Key advancements of MB-105 include:
On-Target Toxicities against normal T-cells: The engineering of MB-105 allows for the preservation of normal T-cells while maintaining cytotoxicity against CD5+ tumor cells, thus reducing the risk of severe immunodeficiency.
Fratricide during CAR T-cell production: The optimized design of the CD5 CAR minimizes self-targeting during production, avoiding T-cell killing that can hinder manufacturing efficiency. This results in a robust production process that reduces costs without complex gene editing.
Minimizing Terminal T-cell Differentiation: Traditional CAR-T cell expansion often leads to early terminal differentiation, producing short-lived effector cells. In contrast, March Biosciences’ proprietary processes preserve minimally differentiated T-cell subsets, promoting increased persistence and enhanced anti-tumor activity in patients.
MB-105 represents an advancement in CAR-T therapies, addressing critical challenges in the treatment of relapsed and refractory CD5 positive T-cell lymphoma. Early clinical results indicate promising safety, efficacy, and durability, with plans to advance into Phase 2 clinical development soon.
Stay updated by visiting oncodaily.com
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
