Agenus Inc. is a biopharmaceutical company and a prominent leader in the field of immuno-oncology, dedicated to advancing treatments for cancer and infectious diseases.
The company’s multifaceted approach to cancer immunotherapy emphasizes combination therapies that leverage a diverse array of antibody therapeutics, adoptive cell therapies, and adjuvants.
Co-founded by Dr. Garo Armen, Chairman and CEO, in 1994 and headquartered in Lexington, Massachusetts, the company initially operated under the name Antigenics.

From its inception, Agenus has been at the forefront of developing innovative immunotherapies, particularly heat shock protein-based cancer vaccines. This pioneering work led to the creation of the Prophage series of personalized anti-cancer vaccines, laying the groundwork for the company’s future endeavors.
In 2000, Antigenics took a significant step by going public on the NASDAQ under the symbol AGEN, enhancing its ability to fund research and development.
That year, the company acquired Aquila Biopharmaceuticals and QS-21 Stimulon, followed by Aronex Pharmaceuticals in 2001.
QS-21 STIMULON is a purified, natural saponin proprietary to Agenus, extensively studied in over 120 clinical trials, consistently demonstrating strong antibody and cell-mediated immune responses along with a favorable safety profile.
This critical component is integral to several vaccines, including GSK’s FDA-approved Shingrix shingles vaccine, which offers over nine years of protection, and the world’s first malaria vaccine, Mosquirix.
As the years progressed, Agenus expanded its focus through strategic collaborations, including a notable partnership with Merck for antibody discovery.
The company has achieved significant clinical advancements with candidates like zalifrelimab (CTLA-4) and balstilimab (PD-1), both entering clinical development in 2016 and 2017.
By 2019, the company was further pushing boundaries with multiple candidates, including botensilimab, a next-generation CTLA-4 immune activator, entering clinical trials.
Over the years, Agenus has built an extensive pipeline of immunological agents, striving to enhance patient access to effective cancer immunotherapy.
Additionally, Agenus is actively developing programs targeting checkpoint modulators CTLA-4, LAG-3 (INCA2385), TIM-3 (INCAGN2390), and CD137.
In 2021, the company made headlines when its subsidiary, MiNK Therapeutics, went public, alongside the launch of SaponiQx to drive innovation in vaccine design and adjuvant manufacturing. These subsidiaries enhance Agenus’s research capabilities and position it as a leader in cancer therapeutics, aimed at improving patient outcomes and quality of life.
By 2022, Agenus was showcasing promising clinical data for its therapies targeting heavily pretreated solid tumors.
In 2023, the company completed enrollment in a Phase 2 trial for its promising botensilimab/balstilimab combination in advanced MSS colorectal cancer and received Fast Track Designation from the U.S. Food and Drug Administration (FDA) for these therapies.
Approximately 1100 patients have been treated with botensilimab across various clinical trial phases, yielding promising results in nine different metastatic cancers.
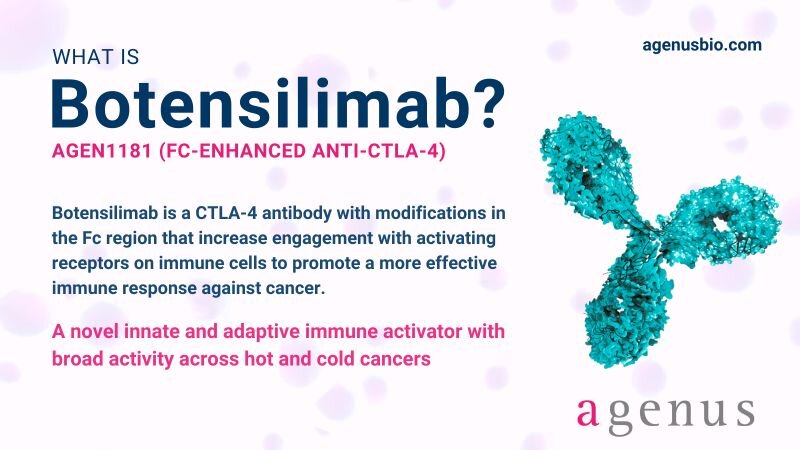
Botensilimab; a next-generation CTLA-4 immune activator
Botensilimab (BOT), also known as AGEN1181, represents a significant advancement in cancer immunotherapy, with an innovative, multifunctional mechanism designed to overcome limitations of first-generation CTLA-4 inhibitors.
CTLA-4 is a key immune checkpoint molecule that acts as a negative regulator of T cell activation. It competes with CD28 for binding to the costimulatory molecules CD80 and CD86 on antigen-presenting cells, thereby inhibiting T cell proliferation and function.
The encouraging efficacy data, coupled with a manageable safety profile, positions botensilimab as a potential game-changer in oncology. It is one of the drugs featured in OncoDaily’s “10 Most Promising Cancer Drugs Not Yet Approved: Solid Tumors – 2024 edition by OncoDaily.”
Recent updates from the NEST-1 clinical trial highlight promising results for neoadjuvant botensilimab combined with balstilimab in treating resectable colorectal cancer.
Among patients with microsatellite stable (MSS) tumors, 67% showed a pathologic response of ≥50%, with two achieving a complete response.
In contrast, all patients with microsatellite instability-high (MSI-H) tumors exhibited a significant pathologic response of 90% or more.
Neoadjuvant BOT/BAL was safe and did not postpone any planned surgeries.
Agenus and their partnerships
Partnerships have been crucial to Agenus’s journey, with the company collaborating with key organizations, including UroGen Pharma, Bristol Myers Squibb, Betta Pharmaceuticals, and others, to sponsor numerous clinical trials.
With a steadfast commitment to innovation and collaboration, Agenus has established itself as a leader in the dynamic field of immune oncology. Since the early 1990s, the company has been at the forefront of advancing this transformative approach, dedicated to improving outcomes for cancer patients worldwide.
Over three decades, Agenus has played a pivotal role in shaping the future of immuno-oncology, continually driving progress and breakthroughs in the fight against cancer.
Looking ahead, Agenus remains focused on expanding its research efforts, forging new partnerships, and advancing its pipeline to deliver innovative therapies that address unmet medical needs in oncology.
Find more information featuring Agenus at oncodaily.com
