
Photo of Aakash Desai from doximity.com
Sep 19, 2024, 11:55
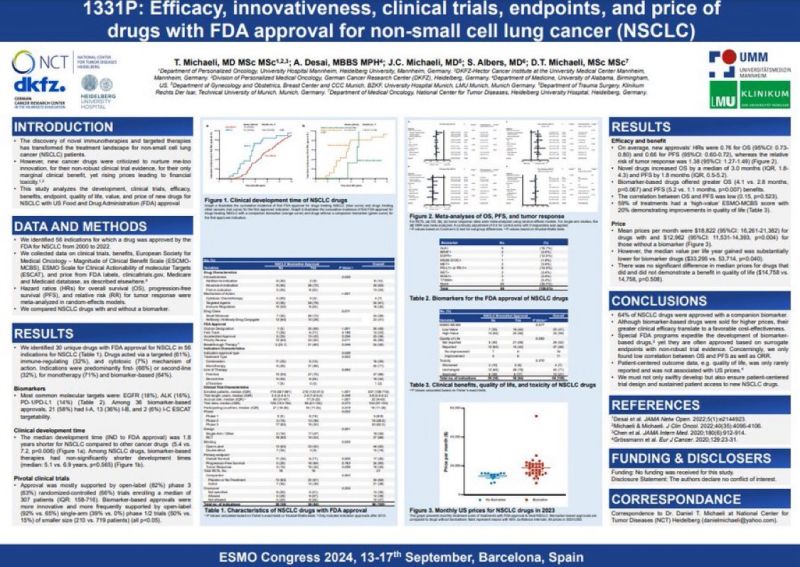
Aakash Desai: Efficacy, Innovation and Cost of FDA-Approved NSCLC Drugs
Aakash Desai, Assistant Professor at O’Neal Comprehensive Cancer Center at UAB, shared on LinkedIn:
“We’re excited to share our poster from ESMO24 on the ‘Efficacy, Innovation and Cost of FDA-Approved NSCLC Drugs’, co-authored with Daniel Michaeli and team.
Key Insights:
- 64% of FDA-approved drugs for NSCLC have biomarker-based targets like EGFR, ALK, and PD-L1.
- The average time from IND to FDA approval is 1.8 years shorter for NSCLC drugs.
- Drugs with biomarkers demonstrated significantly greater overall survival (OS) and progression-free survival (PFS).
- Median monthly cost for NSCLC treatments is $18,822, with biomarker-based therapies often priced higher.
These findings highlight the balance between innovation and cost in lung cancer treatment. Let’s continue working towards more effective and accessible options for patients!”
Source: Aakash Desai/LinkedIn
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
Aug 1, 2025, 19:24
Aug 1, 2025, 19:14
Aug 1, 2025, 18:32
