Jonathan Keats shared on X:
“12 Days of CoMMpass – Day 4: Today we will dive into the nuances in all the numbers in the MMRF CoMMpass study from the perspective of patients and available data. For us TGen Research there have been many recurrent gotchas that its worth a review I believe.
First to be eligible we needed to receive a BM sample with >1% plasma cells by flow cytometry in our central lab before any therapy initiation and the patient needed to start an anti-myeloma therapy with an IMID or PI within 45 days.
Why the 1% plasma cell requirement? It was added after we found 1) most below 1% never started therapy as they didn’t have myeloma. 2) to generate molecular data, at the time we needed more than 250,000 CD138 enriched cells to consistently profile a sample.
Starting with the patient numbers. We actually screened 1954 patients to get the final eligible cohort of 1171 patients after 493 patients were excluded due to low recover or less than 1% plasma cells, and 290 either didn’t have myeloma or didn’t start therapy in 45 days.
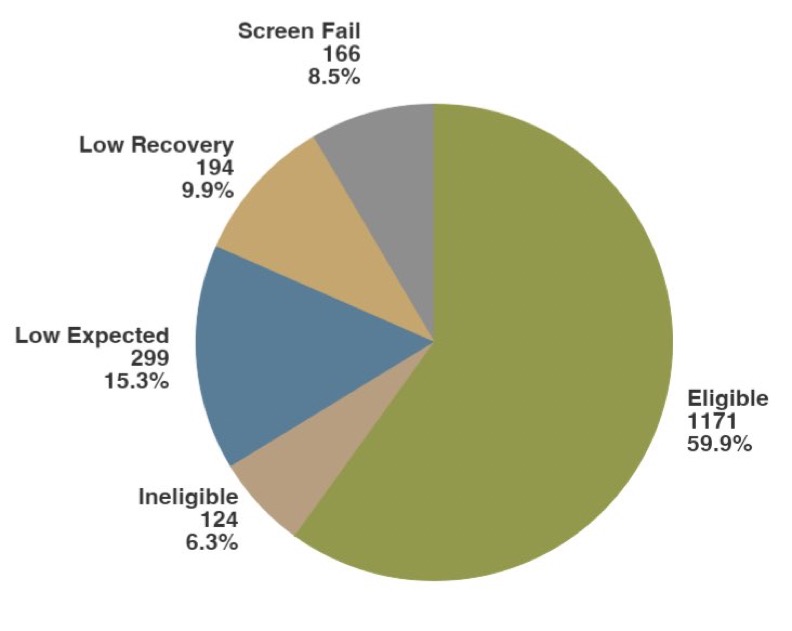
The eligible patient number is one common source of confusion and is often listed as 1143. This nuance is the result the proper CoMMpass cohort and two data sharing agreements, the first with the FORTE study in Italy and the second with the ECOG E1A11 ENDURANCE trail.
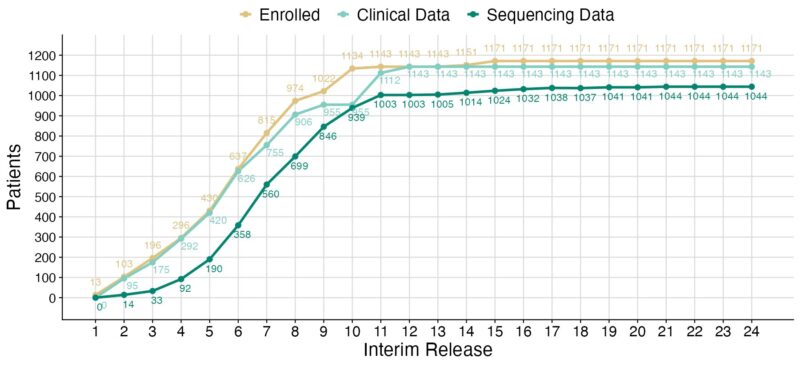
The CoMMpass cohort is actually 971 patients who completed accrual June 2015 and have the full 8 years of observation. We received 172 FORTE patients until June 2016 and an initial tranche of clinical data for all patients but updates stopped after an ASH abstract issue.
Hopefully a final update could be done when FORTE is fully mature… The MMRF, Hearn Jay Cho and Francesca Gay. We continued to accept ENDURNACE patients (n=28) until the study was fully accrued but no demographic or clinical data was integrated into CoMMpass.
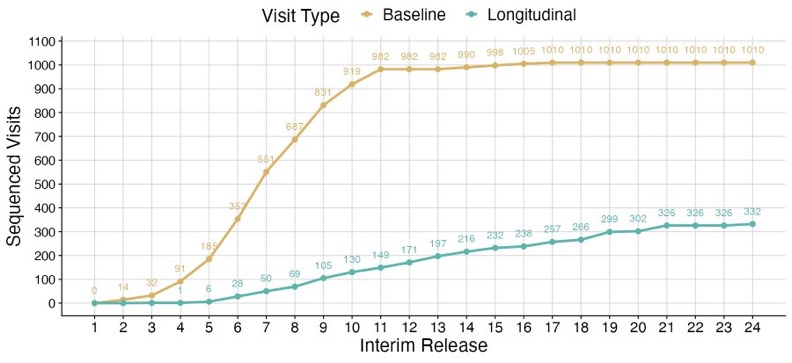
Overall from the cohort of 1171 patients we have ‘some’ clinical data on 1143 and some molecular profiling data on 1044 at some point in their disease course. For molecular profiling data there are 1010 patients with some baseline profiling and 332 longitudinal specimens profiled.
When we look at profiled patients over time there are 169 patients profiled at two timepoint, 39 profiled at 3 timepoints, 10 profiled at 4 timepoints, and 4 with five or more timepoints. The sequential profiling data in CoMMpass is a true differentiator from other efforts.
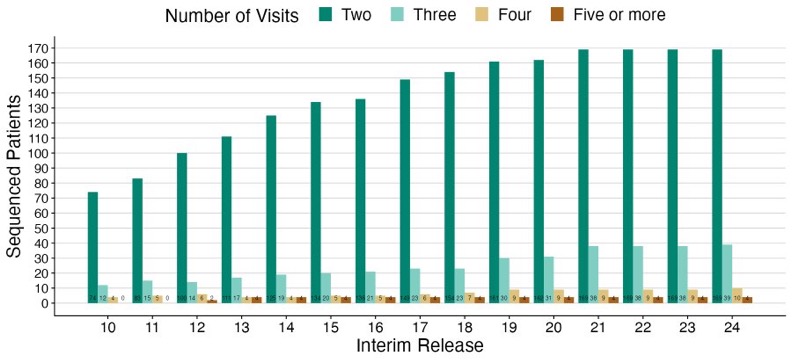
But when we say profiled that doesn’t mean uniformity profiled as not all specimens could be used for DNA and RNA analysis, not all DNA samples could be used for exome and shallow genomes, and sometimes QC requirements were meet for one data type but not another.
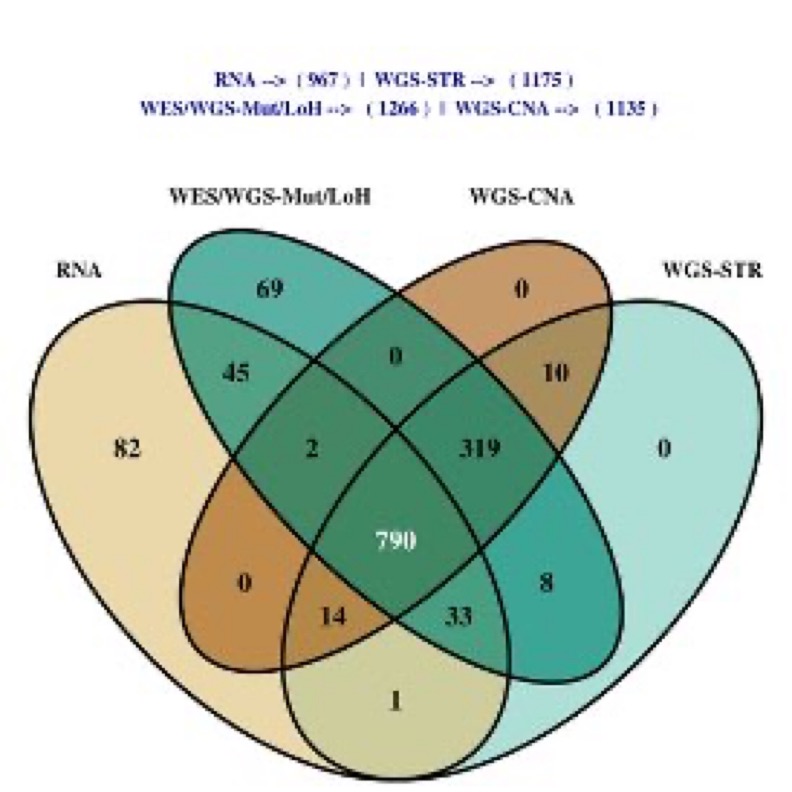
All of these issues can cause issues in analysis if you don’t subset down to the cohort of patients with all the required features.“
Source: Jonathan Keats/X
For more posts by Jonathan Keats about 12 Days of CoMMpass, visit oncodaily.com
Jonathan Keats is an assistant professor at Translational Genomics Reseach Institute (TGen). He specialises in Cancer Genetics, Genome Sequencing, and CGH. Previously, he was a research fellow at Mayo Clinic.
