Quoting Salah-Eddin Al-Batran, Director at The Frankfurt Institute of Clinical Cancer Research, on LinkedIn:
“Friends and colleagues,
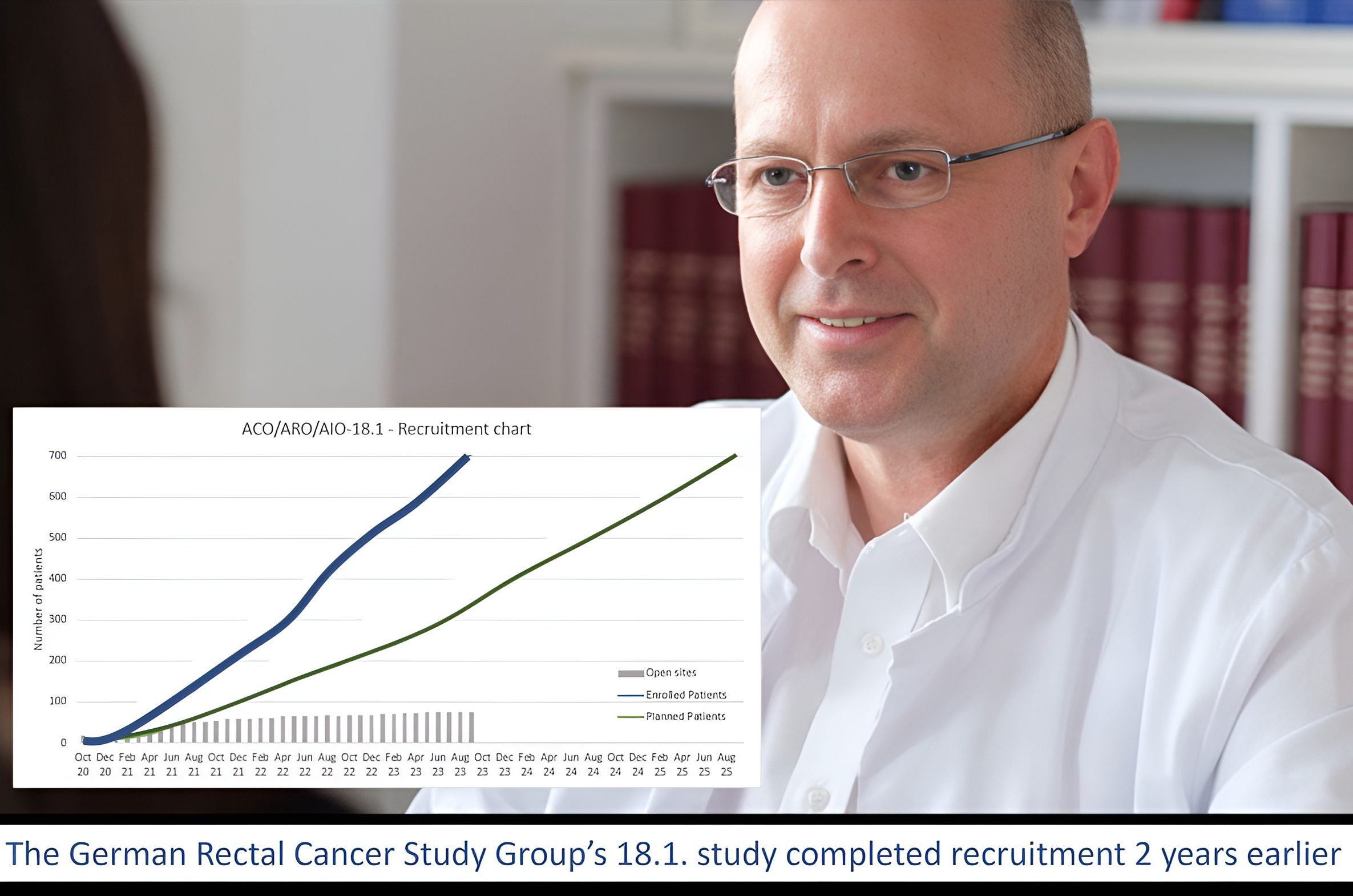
I extend my warmest congratulations to my friends and colleagues Claus Rödel and Emmanouil Fokas, as well as all members, investigators and study nurses of the German Rectal Cancer Study Group, for successfully completing their 18.1 trial. This large trial involved the randomization of 702 patients with rectal cancer, comparing short-course Radiotherapy versus Chemoradiotherapy, followed by Consolidation Chemotherapy, and Selective Organ Preservation for MRI-defined Intermediate and High-risk Rectal Cancer Patients.
The remarkable recruitment achieved in this study serves as an exemplary demonstration of how investigator-initiated research can efficiently enroll patients from complex multidisciplinary trials, emphasizing the significance of academic research groups like those at the University Cancer Center Frankfurt. We take pride in the involvement of our Frankfurt Institute of Clinical Cancer Research in conducting this study.
The ACO/ARO/AIO-18.1 study introduces several novel and innovative elements aimed at optimizing multimodal rectal cancer treatment, building on the foundation laid by the preceding CAO/ARO/AIO-04 and CAO/ARO/AIO-12 randomized trials. These innovations include:
1. Patient selection based on strict, quality-controlled MRI features of intermediate and high-risk characteristics, complementing our ACO/ARO/AIO-18.2 trial for “low-risk” rectal cancer.
2. Chemoradiotherapy regimens incorporating 5-Fluorouracil/oxaliplatin at doses and intensities proven to be effective and well-tolerated without compromising treatment compliance, as seen in CAO/ARO/AIO-04.
3. A treatment sequence involving Cathode Ray Tube, Computed Tomography, and surgery/Watch and Wait adopting the TNT approach established by CAO/ARO/AIO-12 and OPRA trial.
4. Surgical stratification allowing for Watch and Wait management for carefully selected patients showing clinical complete response (cCR).
Hence, the study hypothesizes that Total Neoadjuvant Therapy with 5-Fluorouracil/oxaliplatin-CRT followed by consolidation chemotherapy may increase organ preservation while maintaining Disease-Free Survival (DFS) compared to the RAPIDO-like short-course Radiotherapy followed by consolidation chemotherapy. Looking forward to seeing the results!”
Source: Salah-Eddin Al-Batran/LinkedIn