Vincent Rajkumar shared a post on X:
“FDA approves Dara-VRd for newly diagnosed myeloma.
Quads have become standard of care. Based on 4 phase III trials I recommend Quads for all newly diagnosed patients except those who are frail.
Here is my algorithm for newly diagnosed myeloma transplant eligible.
Open Access article.
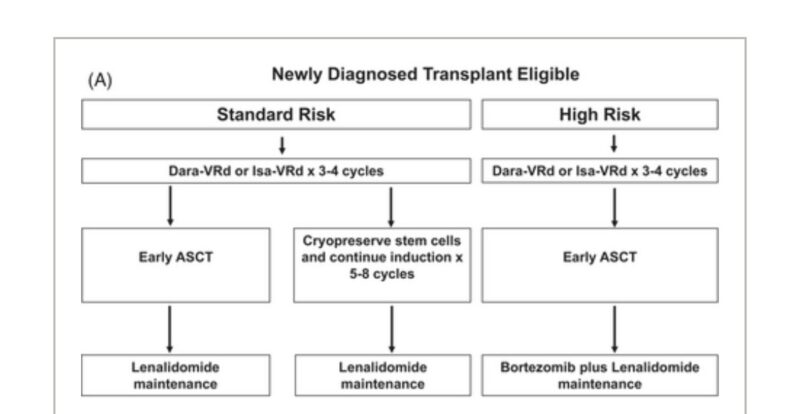
Here is my algorithm for newly diagnosed myeloma transplant ineligible.
Open Access article.
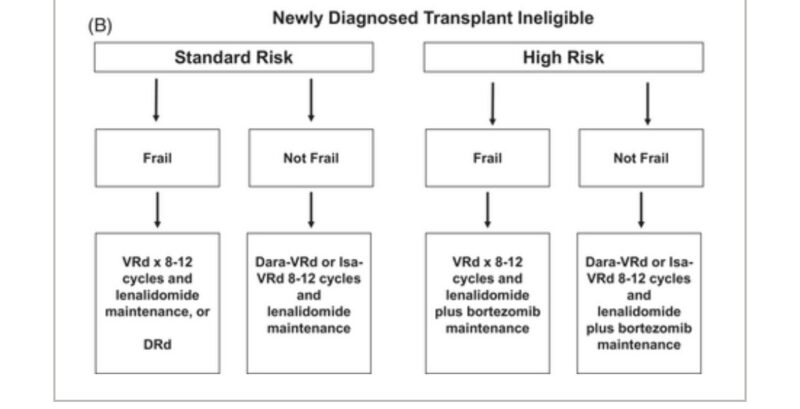
Link to 2024 Update on Diagnosis, Risk Stratification, Prognosis, and Treatment of myeloma.”
Source: Vincent Rajkumar/X
Vincent Rajkumar is a Professor of Medicine at the Mayo Clinic in Rochester, Minnesota, and Chair for the Mayo Clinic Myeloma, Amyloidosis, and Dysproteinemia Group. He also chairs the Board of directors of The International Myeloma Foundation and the Eastern Cooperative Oncology Group (ECOG) Myeloma Committee.
His extensive contributions include over 230 peer-reviewed publications, predominantly focusing on multiple myeloma and related plasma cell disorders. Furthermore, Dr. Rajkumar is a Section Editor for multiple myeloma and related disorders for Leukemia and an Associate Editor for the Mayo Clinic Proceedings.
