Alexander Ariel Padrón González, Internal Clinician at 3cert Limited, shared a post on LinkedIn about a recent paper titled “Innate immune cells: Key players of orchestra in modulating tumor microenvironment (TME)” published in Heliyon Journal.
Authors: Mahvash Sadeghi, Sajad Dehnavi, Moosa Sharifat, Amir Mohammad Amiri, Ali Khodadadi.

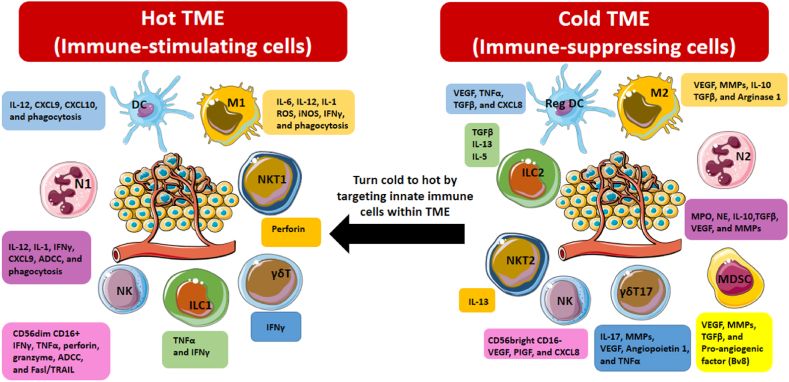
“Innate immune cells modulating tumor microenvironment.
Understanding the mechanisms underlying innate immune cell dysfunction in the TME is essential for developing targeted therapies to unleash their full potential in cancer treatment.
Macrophages: their polarization into M1 or M2 phenotypes influences cancer progression. While M1 macrophages exhibit antitumor activity, M2 macrophages promote tumor growth and metastasis. Understanding the mechanisms driving macrophage polarization and function in the TME could lead to novel therapeutic strategies aimed at repolarizing macrophages to enhance antitumor immunity and reduce metastasis.
Neutrophils: possess both anti- and pro-tumoral functions depending on the tumor stage and type. Their secretion of various mediators contributes to tumor initiation, growth, and metastasis, while also influencing immune responses.
Myeloid derived suppressor cells (MDSCs): promote tumor angiogenesis and progression by suppressing T cell-mediated immune responses. Targeting MDSCs through blockade or depletion strategies presents a promising avenue for cancer therapy.
Dendritic cells (DCs): exhibit diverse functions within the TME, ranging from promoting antitumor responses to facilitating tumor progression. Targeting tumor-associated DCs and manipulating their activity could enhance antitumor immunity and improve cancer therapy outcomes.
Natural killer cells (NK) and natural killer T cells (NKT):
NK cells and NKT cells, important components of the innate immune system, exhibit cytotoxic activity against tumor cells but face challenges within the immunosuppressive TME. Targeting these cells and modulating their activity could enhance their antitumor efficacy and improve cancer immunotherapy outcomes.
Innate lymphoid cells (ILCs): ILCs, a diverse group of innate immune cells, exhibit both pro- and anti-tumoral functions within the TME depending on their subtype and cytokine secretion profile. Targeting specific subsets of ILCs and modulating their activity could offer novel therapeutic strategies for cancer immunotherapy.
Gamma delta (γδ) T lymphocytes: .
γδ T lymphocytes, recognizing stress-induced antigens, play critical roles in tumor immunity but face challenges within the immunosuppressive TME. Targeting γδ T cells and modulating their activity could enhance their antitumor efficacy and improve cancer therapy outcomes.
Effect of antibody drug conjugates (ADCs) on innate immune cells:
ADCs, targeting specific antigens on tumor cells, can activate innate immune cells such as NK cells and macrophages, enhancing their cytotoxic activity against cancer cells. Understanding the mechanisms by which ADCs interact with innate immune cells in the TME could lead to the development of more effective cancer therapies. Harnessing the potential of ADCs to engage innate immune cells offers promising opportunities for improving cancer treatment outcomes.”