
Kristina Jenei: Are countries harmonizing up or down?
Dario Trapani, Medical Oncologist at European Institute of Oncology, shared a post by Kristina Jenei, Associate Editor at the Journal of Cancer Policy, on X:
“Fresh new air with the SUPER Kristina Jenei and Vinay Prasad on Orbis , HTA and the lack of clear added value.”
Quoting Kristina Jenei‘s post:
“Our article out today in The Lancet Oncology
Are countries harmonizing up or down?
We examine the clinical benefit, HTA review times, and prices of drugs reviewed through Project Orbis.

We found compared to other FDA approvals, OS and PFS gains were not significantly different.
Yes.. lots of immunotherapies where a subset of patient may be high responders – but we don’t know how these drugs are chosen.
FDA says ‘clinically significant and promising’
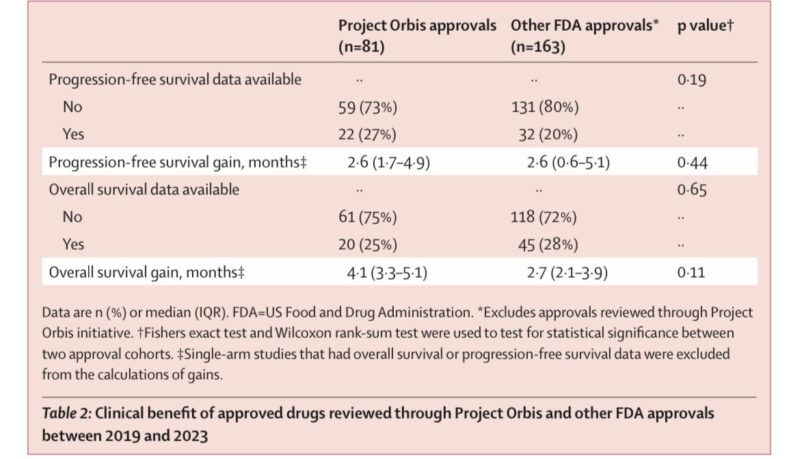
HTA orgs are not recommending all drugs for reimbursement and the review times are getting longer. More complicated applications?
CADTH recommended 72%
NICE: 40% (pos)
SMC: 100% Here’s some of the rationales with the negative recommendations. Too much uncertainty?
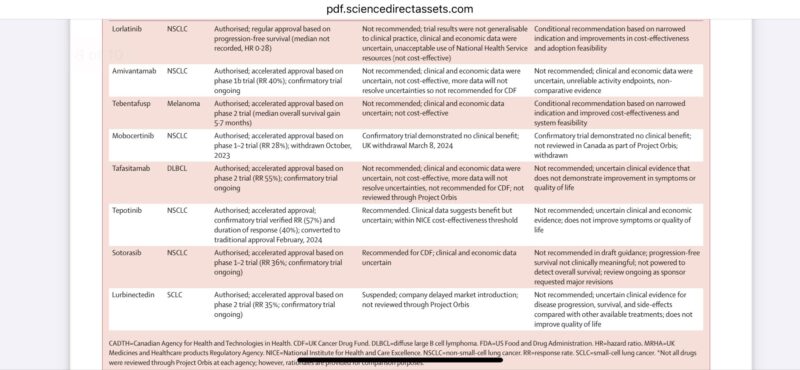
list prices were 20K a month. Even with 50% discounts, we are talking about huge strain for patients and public health systems. When regulatory approvals speed up, HTA review got slower. What will this mean for public health systems?
Project Orbis builds on a long history of international collab.
Bringing good drugs to patients faster is key, but as a whole, it’s unclear whether this happening.
We don’t know how drugs are selected. Transparent governance is key as this program expands.”
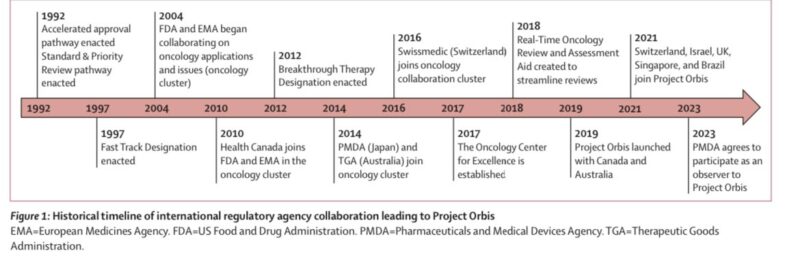
Source: Dario Trapani/X and Kristina Jenei/X
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
