The PALOMA-3 trial was designed to address a growing and clinically relevant challenge in modern oncology: how to deliver effective targeted therapies in a manner that maintains clinical benefit while reducing treatment burden for patients and healthcare systems. As treatment for EGFR-mutated non–small cell lung cancer (NSCLC) increasingly relies on long-term combination regimens, the route and logistics of drug administration have become critical determinants of real-world feasibility.
Amivantamab is a bispecific antibody targeting EGFR and MET that has demonstrated meaningful clinical activity in EGFR-mutated NSCLC, particularly in combination with Lazertinib. While intravenous (IV) amivantamab has become an important component of the therapeutic landscape, IV delivery is associated with prolonged infusion times, intensive monitoring requirements, and a high incidence of infusion-related reactions.
To address these limitations, a subcutaneous formulation, Rybrevant FASPRO, was developed with the aim of delivering comparable drug exposure in a significantly more convenient format. The PALOMA-3 trial was undertaken to rigorously evaluate whether subcutaneous amivantamab plus lazertinib could match IV amivantamab-based regimens in pharmacokinetics, while also exploring clinical outcomes, safety, patient experience, and healthcare resource utilization.
Study Design and Objectives
PALOMA-3 is a randomized, controlled clinical pharmacology study comparing subcutaneous Rybrevant FASPRO plus lazertinib with intravenous amivantamab-based regimens plus lazertinib. The study was primarily designed to support formulation bridging, a critical regulatory step required to enable broader clinical use of the subcutaneous formulation.
The trial incorporated two co-primary pharmacokinetic endpoints, both assessed at steady state: amivantamab Ctrough, reflecting trough serum concentration prior to dosing, and area under the concentration–time curve (AUC), representing overall systemic exposure. These endpoints are widely accepted by regulatory authorities as the key metrics for demonstrating pharmacokinetic comparability between formulations of biologic agents.
Secondary and exploratory endpoints included safety and tolerability, administration-related outcomes, objective response rate (ORR), progression-free survival (PFS), overall survival (OS), patient-reported experience measures, and healthcare resource utilization.
Pharmacokinetic Outcomes
The PALOMA-3 trial met both co-primary pharmacokinetic endpoints, demonstrating comparable amivantamab exposure between subcutaneous and intravenous formulations. Steady-state Ctrough values for subcutaneous Rybrevant FASPRO fell within predefined equivalence margins relative to IV amivantamab, confirming consistent maintenance of therapeutic drug concentrations across the dosing interval.
Similarly, AUC analyses showed that overall systemic exposure achieved with subcutaneous administration was comparable to that of the IV formulation. These findings establish pharmacokinetic equivalence and provide a robust foundation for extrapolating the established efficacy and safety of IV amivantamab to the subcutaneous formulation.
Additional pharmacokinetic parameters, including peak concentration and time to peak exposure, differed as expected between subcutaneous and intravenous delivery but did not indicate any clinically meaningful disadvantage for the subcutaneous route.
Clinical Efficacy Results
Beyond pharmacokinetic comparability, PALOMA-3 generated clinically informative efficacy data. Overall survival analyses demonstrated a statistically significant improvement in survival with subcutaneous therapy, with a 38% reduction in the risk of death compared with IV amivantamab-based regimens (hazard ratio 0.62; nominal P=0.02). At 12 months, 65% of patients treated with the subcutaneous regimen were alive, compared with 51% in the intravenous arm, suggesting a meaningful survival advantage favoring subcutaneous administration.
Tumor response outcomes further supported the clinical comparability of the two formulations. The objective response rate with subcutaneous Rybrevant FASPRO plus lazertinib was 30%, meeting criteria for noninferiority relative to the 33% ORR observed with IV amivantamab. These findings indicate that altering the route of administration does not compromise antitumor activity.
Progression-free survival analyses showed a numerically longer median PFS for the subcutaneous regimen, reaching 6.1 months, compared with 4.3 months for intravenous therapy. Although this difference did not reach formal statistical significance (P=0.20), the direction of effect was consistent with the overall efficacy profile favoring subcutaneous delivery.
Read About Rybrevant FASPRO Approval on OncoDaily
Safety and Tolerability
The safety profile observed in PALOMA-3 was consistent with the known toxicities of amivantamab and lazertinib, with no new or unexpected safety signals identified for the subcutaneous formulation. A particularly notable finding was the marked reduction in infusion-related reactions (IRRs). IRRs occurred in 13% of patients receiving subcutaneous therapy, compared with 66% of those treated with intravenous amivantamab, representing an approximately five-fold reduction.
Rates of venous thromboembolism (VTE) were also lower in the subcutaneous arm, occurring in 9% of patients, compared with 14% in the IV arm. While exploratory, this difference further supports the favorable safety profile of the subcutaneous formulation. Overall treatment discontinuation rates due to adverse events were low and comparable between treatment arms, supporting the feasibility of long-term administration in patients with EGFR-mutated NSCLC.
Patient Experience and Healthcare Resource Utilization
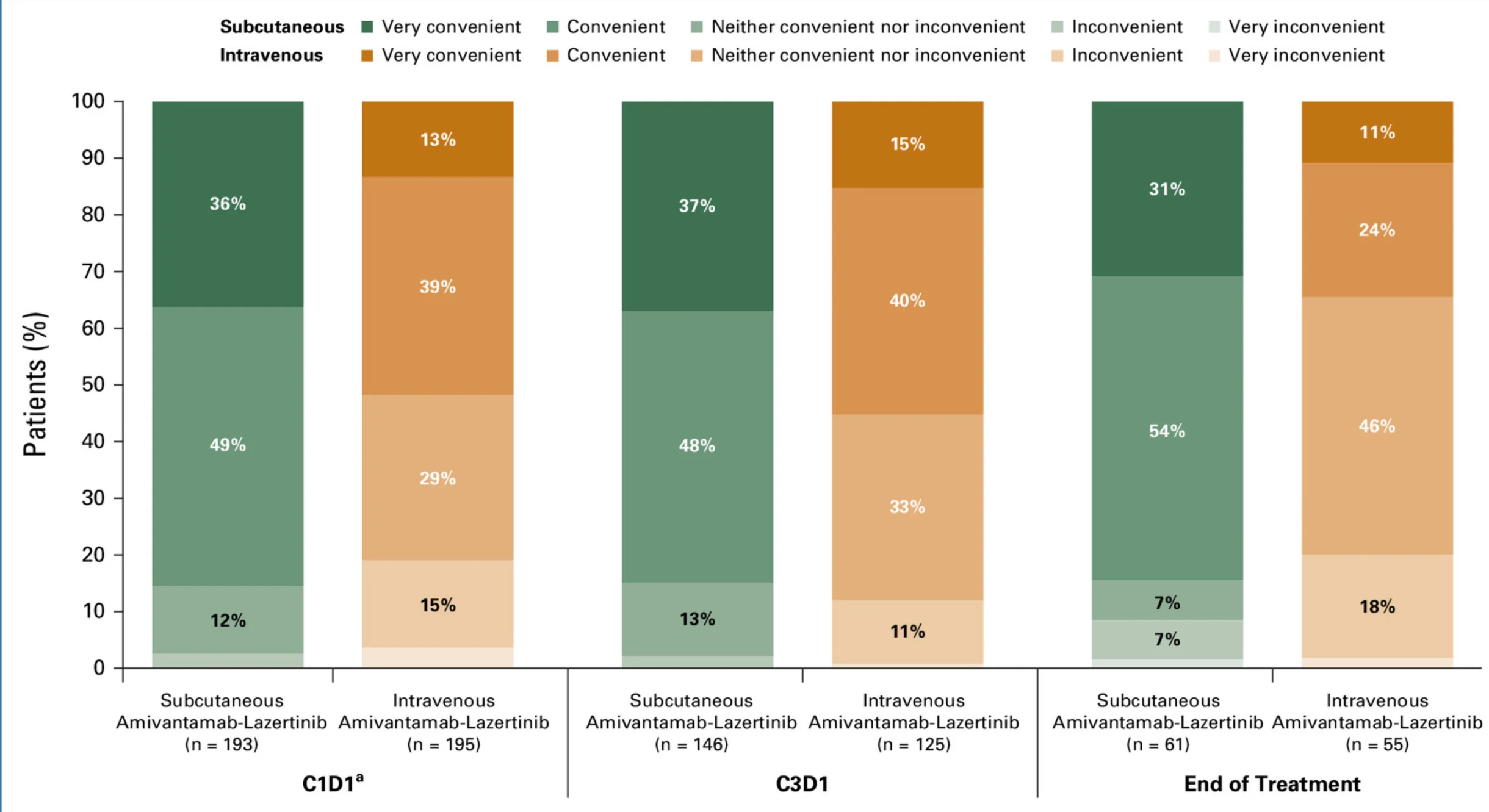
One of the most clinically impactful aspects of PALOMA-3 was the demonstration of substantial improvements in patient experience and healthcare efficiency. Subcutaneous administration reduced treatment time from approximately five hours with IV infusion to roughly five minutes per injection.
On day one of therapy, median time-in-chair decreased from 6.5 hours in the IV arm to just 23 minutes in the subcutaneous arm, reflecting a transformative reduction in clinic occupancy. These operational gains translated into improved patient perception, with 85% of patients in the subcutaneous arm reporting treatment as convenient, compared with 52% in the IV arm.
Patient preference strongly favored subcutaneous delivery, with approximately 81% of patients indicating they preferred the subcutaneous injection over historical IV therapies and would recommend it. In parallel, subcutaneous administration significantly reduced active healthcare professional time, alleviating staffing demands and improving clinic throughput.
Clinical and Regulatory Implications
The PALOMA-3 trial demonstrates that subcutaneous Rybrevant FASPRO plus lazertinib not only achieves pharmacokinetic equivalence with IV amivantamab-based regimens but also delivers meaningful advantages in safety, patient experience, and healthcare resource utilization. The observed improvements in overall survival, combined with noninferior response rates and reduced administration-related toxicity, provide a compelling rationale for transitioning to subcutaneous delivery where appropriate.
From a regulatory perspective, the robust pharmacokinetic data generated by PALOMA-3 support formulation bridging and enable broader clinical adoption of the subcutaneous formulation. From a clinical standpoint, the trial highlights the importance of delivery innovation as an integral component of therapeutic advancement.
Conclusion
The PALOMA-3 trial establishes subcutaneous Rybrevant FASPRO plus lazertinib as a pharmacokinetically equivalent, clinically effective, and operationally superior alternative to intravenous amivantamab-based regimens. By combining comparable drug exposure with improved survival outcomes, reduced infusion-related toxicity, and substantial gains in patient convenience and healthcare efficiency, PALOMA-3 represents a meaningful step forward in the evolution of patient-centered targeted therapy for EGFR-mutated NSCLC.