At ESMO 2025 in Berlin, Zhenhua Liu from Chengdu, China, presented phase 3 results from the randomized, open-label FRUSICA-2 trial (NCT05522231). The talk reviewed the efficacy and safety of FRUQ+SIN versus investigator-selected AXI/EVE as second-line therapy after one prior VEGFR-TKI in advanced or metastatic RCC. This article summarizes the key data and discussion points from that presentation.
Introduction
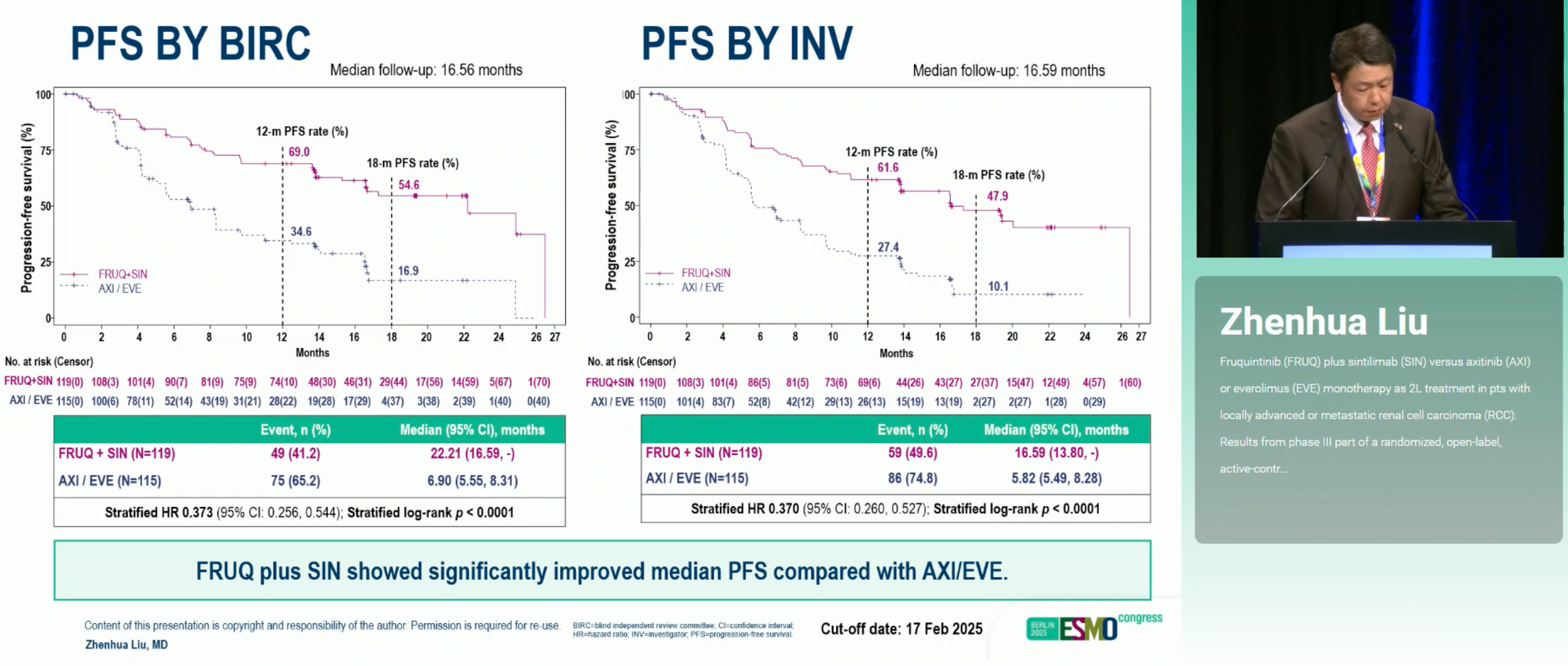
The randomized, open-label FRUSICA-2 trial (NCT05522231) evaluated Fruquintinib plus Sintilimab against investigator-selected monotherapy (axitinib [AXI] or everolimus [EVE]) as second-line treatment for patients with locally advanced or metastatic renal cell carcinoma (RCC) previously exposed to one VEGFR-TKI. In the phase 3 readout (data cut-off Feb 17, 2025; median follow-up 16.56 months), FRUQ+SIN delivered a marked improvement in blinded independent review committee (BIRC)–assessed progression-free survival (PFS) and objective response rate (ORR), with durable responses and acceptable tolerability.
Study Design
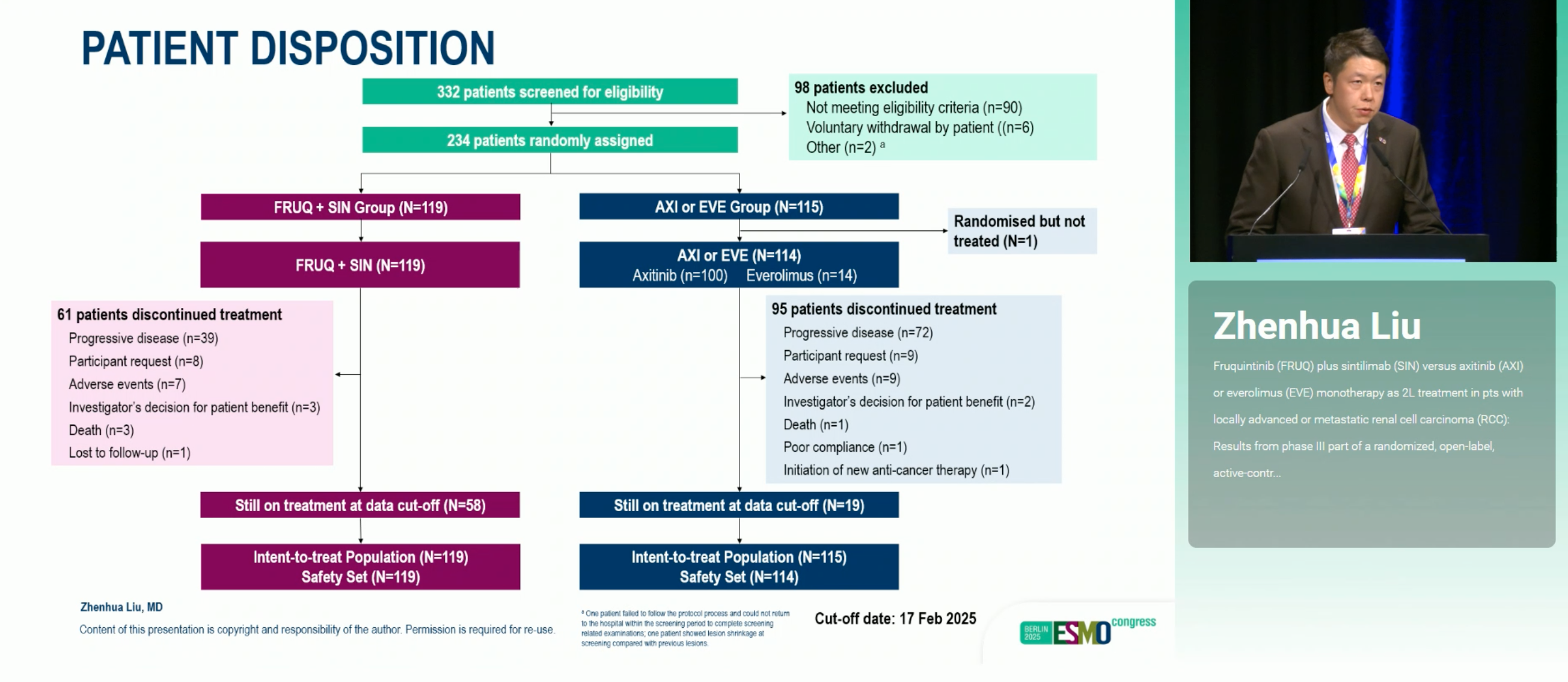
FRUSICA-2 enrolled 234 patients (Oct 2022–Dec 2023) and randomized them 1:1 to FRUQ+SIN or AXI/EVE in 21-day cycles, stratified by ECOG performance status and IMDC risk. The primary endpoint was BIRC-assessed PFS per RECIST 1.1; key secondary endpoints included ORR, duration of response (DOR), overall survival (OS), and safety.
Population: Advanced/metastatic RCC after one prior VEGFR-TKI
Arms: 1:1, by ECOG PS and IMDC risk
- Fruquitinib+Sintilimab: FRUQ 5 mg QD (2 weeks on/1 week off) + SIN 200 mg IV Q3W
- Investigator’s choice Axitinib 5 mg BID or Everolimus 10 mg QD
Disposition: 119 to FRUQ+SIN; 115 to AXI/EVE (101 received AXI; 1 untreated)
Data cut-off: Feb 17, 2025; median follow-up 16.56 months
Results
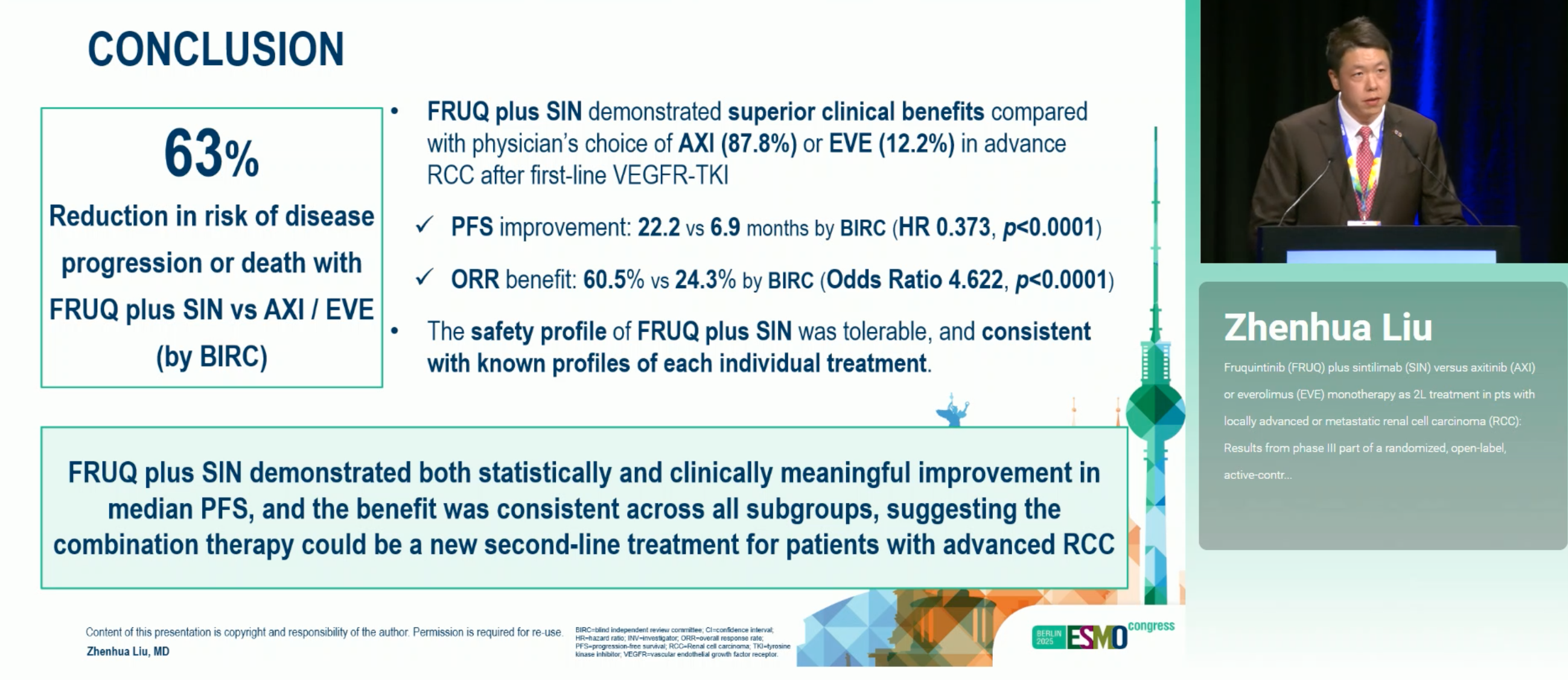
FRUQ+SIN significantly improved efficacy vs AXI/EVE: mPFS 22.21 vs 6.90 months (HR 0.373; p<0.0001), with higher ORR 60.5% vs 24.3% and longer DOR 23.69 vs 11.33 months. Benefits were consistent across IMDC risk groups (strongest in favorable/intermediate). OS is immature (~20% events). Safety was manageable: more grade ≥3 TEAEs, but fatal TEAEs were comparable.
- Primary (BIRC PFS): 22.21 vs 6.90 mo; HR 0.373; p<0.0001
- ORR: 60.5% vs 24.3% (OR 4.622; p<0.0001)
- DOR: 23.69 vs 11.33 mo
- Subgroups: Benefit across IMDC; strongest in favorable/intermediate
- OS: Not mature (~20% data)
- Safety: ↑ grade ≥3 TEAEs; fatal TEAEs similar (4.2% vs 4.4%)
Key Take-Home Messages
- FRUQ+SIN tripled PFS vs AXI/EVE and >2× ORR, with long DOR.
- Benefits observed across IMDC risk groups, strongest in favorable/intermediate risk.
- Median DOR ~24 months supports depth and persistence of response.
- Higher grade ≥3 events with combo, but fatal TEAEs comparable to control.
- Survival data not mature; continued follow-up will clarify magnitude of OS benefit.
- For patients post-VEGFR-TKI, FRUQ+SIN represents a highly effective, tolerable option.
You Can Follow ESMO 2025 live by checking the official program, abstracts, and announcements on the ESMO website, and get rolling highlights, summaries, expert takes, and Special Live Coverage on OncoDaily