When the CLEOPATRA trial results were first presented, they did more than show a statistical improvement. They reset expectations.
Before CLEOPATRA, HER2-positive metastatic breast cancer was considered aggressive but manageable. After CLEOPATRA, it became a disease where long-term survival was realistic, even in the metastatic setting.
More than a decade later, the CLEOPATRA trial remains one of the most practice-defining studies in modern oncology. It did not introduce the first HER2 therapy. That was trastuzumab. It did not introduce the first dual blockade concept. But it proved, beyond doubt, that intensifying HER2 targeting at the right moment could dramatically extend life.
And in doing so, the CLEOPATRA trial became the backbone of first-line therapy worldwide and the benchmark against which future HER2 therapies would be measured.

The Clinical Context Before CLEOPATRA
Trastuzumab transformed HER2-positive breast cancer outcomes in both early and metastatic settings. However, resistance inevitably developed, and median overall survival in metastatic disease remained limited even in the trastuzumab era.
The biological rationale for dual HER2 blockade was compelling. HER2 signaling depends heavily on receptor dimerization, particularly with HER3. Blocking HER2 at multiple extracellular domains could theoretically prevent pathway escape.
Pertuzumab binds to a different epitope of HER2 than trastuzumab and inhibits ligand-dependent HER2 dimerization, particularly HER2–HER3 interactions. Combining the two antibodies offered a mechanistically elegant solution.
But whether this approach would translate into improved survival remained uncertain.
CLEOPATRA answered that question definitively.
CLEOPATRA Trial Design
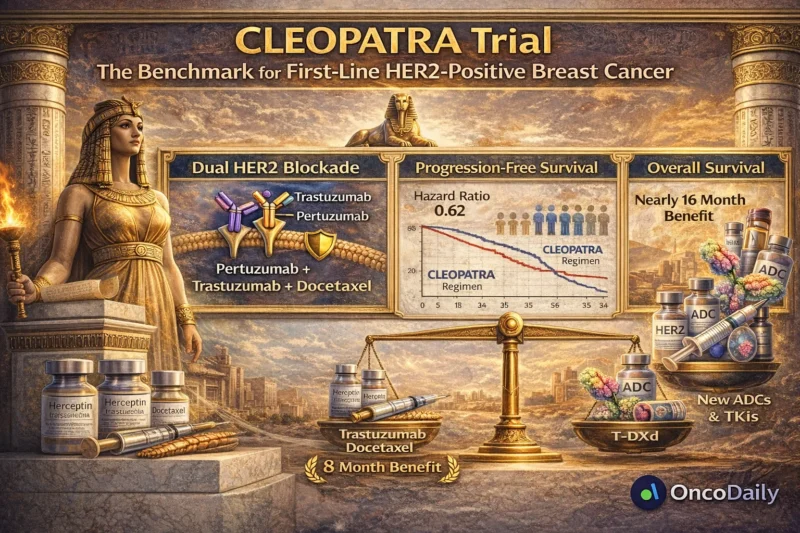
The CLEOPATRA trial was a randomized, double-blind, phase III study evaluating pertuzumab, trastuzumab, and docetaxel versus trastuzumab and docetaxel alone in previously untreated HER2-positive metastatic breast cancer.
The primary endpoint was progression-free survival (PFS), with overall survival (OS) as a key secondary endpoint.
The study enrolled 808 patients and was published in the New England Journal of Medicine in 2012 (Baselga et al., 2012, N Engl J Med).
At the time, demonstrating a meaningful survival advantage in metastatic breast cancer was challenging. Few therapies had shifted the curve dramatically.
CLEOPATRA did.
The PFS Breakthrough
The addition of pertuzumab significantly improved median progression-free survival:
- 18.5 months in the pertuzumab arm
- 12.4 months in the control arm
- Hazard ratio 0.62
This was not incremental. It was transformative.
For the first time, dual HER2 blockade upfront delayed progression by over six months compared with single HER2 targeting. But the true impact of CLEOPATRA became clear with overall survival.
The Survival Data That Changed Practice
Updated overall survival results demonstrated a median OS of:
- 56.5 months in the pertuzumab group
40.8 months in the control group
That is an improvement of nearly 16 months, a magnitude rarely seen in metastatic solid tumors (Swain et al., 2015, N Engl J Med).
Five-year survival approached 50%. This was unprecedented.
For a disease historically associated with rapid progression, patients were now living close to five years on average. The CLEOPATRA trial did not just improve statistics. It redefined prognosis.
Why CLEOPATRA Worked
The success of CLEOPATRA was rooted in biology.
HER2-positive tumors are addicted to HER2 signaling. Trastuzumab blocks ligand-independent HER2 activation and mediates antibody-dependent cellular cytotoxicity. Pertuzumab blocks HER2 dimerization with HER3, one of the most potent oncogenic signaling pathways via PI3K/AKT.
The dual blockade prevents compensatory escape. In addition, docetaxel provided cytotoxic synergy. This multi-pronged attack created durable control.
The concept of dual HER2 blockade became the standard not only in metastatic disease but also in early-stage settings (as later shown in trials such as APHINITY; von Minckwitz et al., 2017, N Engl J Med).
Safety and Tolerability
One of the most important aspects of CLEOPATRA was that survival gains did not come at the cost of prohibitive toxicity.
Rates of cardiotoxicity a major concern with HER2-targeted therapy, were not significantly increased with the addition of pertuzumab.
Adverse events were manageable and consistent with docetaxel-based chemotherapy. This safety profile enabled rapid global adoption.
CLEOPATRA’s Long-Term Impact on the Treatment Landscape
For more than a decade, the first-line standard of care for HER2-positive metastatic breast cancer remained the CLEOPATRA regimen: trastuzumab, pertuzumab, and a taxane.
This durability speaks volumes. Even in the era of antibody–drug conjugates and novel tyrosine kinase inhibitors, CLEOPATRA remained the benchmark regimen in the frontline setting and the foundation upon which modern treatment sequencing was built.
Did CLEOPATRA Cure Metastatic HER2-Positive Disease?
Despite impressive survival improvements, metastatic disease remains incurable in most cases.
However, CLEOPATRA extended survival to a point where HER2-positive metastatic breast cancer increasingly resembles a chronic disease for many patients. Modern treatment strategies rely on sequential HER2-directed therapies, a model that became possible because CLEOPATRA provided durable disease control in the first line.
A Trial That Changed Expectations
Perhaps the most important legacy of CLEOPATRA is psychological.
Before CLEOPATRA, improving median survival by several months was considered meaningful. After CLEOPATRA, clinicians began expecting double-digit survival gains.
It shifted the benchmark. Future trials would now be judged against a new standard. In oncology, that is the mark of a practice-changing study.
Lessons from CLEOPATRA
- Target biology precisely and early.
- Combine agents with complementary mechanisms.
- Measure overall survival, not just progression.
- Maintain tolerability while intensifying therapy.
- These principles now guide HER2 drug development.
CLEOPATRA in the Era of Antibody–Drug Conjugates
The development of antibody–drug conjugates introduced a new era of HER2-directed therapy. Among these agents, trastuzumab deruxtecan demonstrated unprecedented efficacy in previously treated HER2-positive metastatic breast cancer and rapidly became the preferred second-line therapy.
More recently, the phase III DESTINY-Breast09 trial evaluated trastuzumab deruxtecan in combination with pertuzumab in the first-line setting. The study demonstrated a significant improvement in progression-free survival compared with the CLEOPATRA regimen, with median progression-free survival extending beyond three years.
These results have introduced a new potential frontline treatment option and have been incorporated into some clinical guidelines. However, overall survival data remain immature, and CLEOPATRA continues to represent the longest-established survival benchmark in first-line HER2-positive metastatic breast cancer.
Rather than replacing CLEOPATRA, these developments build upon the foundation it established.

CLEOPATRA Today
Even as new therapies emerge and treatment strategies evolve, CLEOPATRA remains one of the most influential trials in oncology history. It established dual HER2 blockade, transformed survival expectations, and defined the structure of modern HER2-directed therapy.
More than a decade later, we are still practicing in the world CLEOPATRA created. And that is the definition of a landmark trial.
Written by Armen Gevorgyan, MD


