The AMPLITUDE trial represents a major step forward in the evolution of precision medicine for prostate cancer, specifically addressing whether PARP inhibition can improve outcomes when introduced early in the disease course. While PARP inhibitors have already demonstrated clinical benefit in metastatic castration-resistant prostate cancer (mCRPC), their role in metastatic castration-sensitive prostate cancer (mCSPC) has remained uncertain.
Biologically, mCSPC offers a compelling opportunity for therapeutic intensification. Tumors are less genomically complex, patients have better performance status, and treatment resistance mechanisms are less entrenched. Importantly, patients with homologous recombination repair (HRR) gene alterations represent a molecularly defined subgroup with inherent defects in DNA damage repair, rendering them particularly vulnerable to PARP inhibition.
The AMPLITUDE trial was therefore designed to test whether combining the PARP inhibitor Niraparib with standard androgen receptor pathway inhibition using Abiraterone acetate and prednisone could meaningfully improve outcomes in patients with HRR-mutated mCSPC.
Study Design and Methods
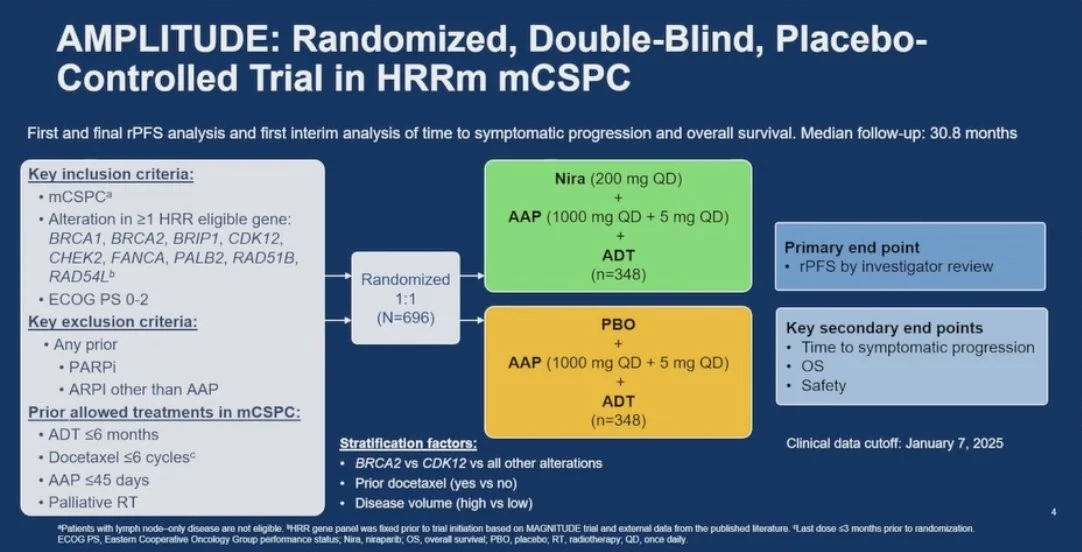
AMPLITUDE (ClinicalTrials.gov Identifier: NCT04497844) is an international, randomized, double-blind, placebo-controlled phase III study. The trial enrolled patients with newly diagnosed mCSPC who had confirmed deleterious alterations in HRR genes, including BRCA1, BRCA2, ATM, PALB2, and others involved in homologous recombination repair.
Eligible patients were randomized to receive niraparib plus abiraterone acetate and prednisone (AAP) or placebo plus AAP, with all patients receiving continuous androgen deprivation therapy. Randomization was stratified according to disease volume, geographic region, and specific HRR gene alteration to ensure balanced baseline characteristics.
The primary endpoint of the AMPLITUDE trial was radiographic progression-free survival (rPFS), assessed by blinded independent central review according to RECIST v1.1 and PCWG3 criteria. Key secondary endpoints included overall survival (OS), time to symptomatic progression, time to subsequent systemic therapy, safety, and health-related quality of life.
Efficacy Results
The AMPLITUDE trial met its primary endpoint, demonstrating a statistically significant and clinically meaningful improvement in radiographic progression-free survival with niraparib plus AAP compared with AAP alone in patients with HRR-altered mCSPC. Treatment intensification with PARP inhibition resulted in an early and sustained separation of rPFS curves, indicating durable disease control in this molecularly selected population.
The magnitude of benefit was greatest among patients with BRCA1 or BRCA2 mutations, who represent the subgroup most sensitive to PARP inhibition. In this cohort, median rPFS was approximately 16.6 months with niraparib plus AAP, compared with 10.9 months with AAP alone, corresponding to a 47% reduction in the risk of radiographic progression or death (hazard ratio 0.53). This robust benefit highlights the biological synergy between androgen receptor pathway suppression and PARP inhibition in BRCA-mutated disease.
Importantly, rPFS improvement was not confined to BRCA-mutated tumors. In the overall HRR-altered population, which included patients with alterations in additional DNA repair genes, niraparib plus AAP continued to demonstrate superior disease control. Median rPFS in this broader cohort was approximately 16.5 months versus 13.7 months for the control arm, supporting the relevance of comprehensive genomic profiling beyond BRCA testing alone.
Beyond the primary endpoint, clinically meaningful improvements were also observed across several secondary endpoints. Patients receiving niraparib plus AAP experienced a delay in time to symptomatic progression, reflecting prolonged maintenance of clinical well-being, and a longer time to initiation of chemotherapy, an outcome of particular importance in mCSPC where deferring cytotoxic treatment remains a key therapeutic objective. Overall survival data are currently immature, and continued follow-up is ongoing to determine whether early rPFS benefits translate into long-term survival advantage.
Safety and Tolerability
The safety profile observed in AMPLITUDE was consistent with the known adverse event profiles of niraparib and abiraterone. Hematologic toxicities, including anemia and thrombocytopenia, occurred more frequently in the niraparib arm, reflecting class-specific PARP inhibitor effects. Non-hematologic adverse events included fatigue, nausea, hypertension, and elevations in liver enzymes.
Grade ≥3 adverse events were higher with the combination therapy; however, treatment discontinuation rates remained acceptable, and most toxicities were manageable with dose interruptions, reductions, or supportive care. No unexpected safety signals emerged, an important consideration given the earlier disease stage and longer anticipated treatment duration in mCSPC.
Quality-of-life analyses indicated that rPFS improvement was not offset by disproportionate deterioration in patient-reported outcomes, reinforcing the clinical relevance of the efficacy findings.
Clinical Implications
The AMPLITUDE trial has important implications for contemporary prostate cancer management. First, it reinforces the concept that molecular stratification should guide treatment decisions even in hormone-sensitive disease. Routine testing for HRR gene alterations at the time of metastatic diagnosis is likely to become increasingly important.
Second, AMPLITUDE suggests that earlier introduction of PARP inhibition may provide greater disease control than reserving these agents for later, castration-resistant stages. This strategy aligns with broader oncology trends favoring early intensification for biologically high-risk subgroups.
Finally, the trial distinguishes itself from prior studies by demonstrating benefit in a strictly biomarker-defined population, avoiding overtreatment of patients unlikely to benefit and setting a benchmark for precision-driven trial design.

Read About AKEEGA Approval on OncoDaily
Positioning Within the Niraparib Development Program
AMPLITUDE complements earlier findings from the MAGNITUDE trial, which evaluated the same combination in mCRPC. Together, these trials map a coherent development pathway for niraparib across the prostate cancer continuum, from hormone-sensitive to castration-resistant disease, with HRR mutation status as the central determinant of benefit.
Conclusion
The AMPLITUDE trial establishes niraparib plus abiraterone as an effective, biomarker-driven treatment strategy for patients with HRR-mutated mCSPC. By significantly improving radiographic progression-free survival and demonstrating manageable safety in an earlier disease setting, AMPLITUDE advances the paradigm of precision oncology in prostate cancer and provides a strong rationale for integrating PARP inhibition upfront in selected patients.