Mia Hamant was a remarkable goalkeeper for the University of Washington women’s soccer team and a dedicated student-athlete. Known for her outstanding skills on the field, she played a pivotal role in the team’s defense, ranking among the best goalkeepers nationally in 2024. Beyond athletics, she excelled academically, embodying perseverance and dedication. Her significance in soccer was matched by her courage in battling a rare and aggressive form of stage 4 kidney cancer, diagnosed in April 2025.

Tragically, Mia passed away on November 6, 2025, at the age of 21. Her fight against cancer, openness about her journey, and indomitable spirit inspired teammates, coaches, and the wider community, leaving a lasting legacy of strength and hope.
Early Life and Soccer Career of Mia Hamm
Mia Hamant hailed from Corte Madera, California, where she demonstrated athletic talent from a young age. She led her club team, Marin FC, to two NorCal State Cup titles and played soccer and softball at Redwood High School, earning All-League First Team honors for three years and being named team MVP in her senior year. In high school, she also served as team captain and was recognized as Defensive Player of the Year.
At the University of Washington, Mia was a standout goalkeeper for the Huskies women’s soccer team. After redshirting her freshman year due to an ACL injury, she made a strong comeback. In the 2024 season, Mia was one of the best goalkeepers in the Big Ten Conference, recording a 0.882 save percentage (third nationally), seven clean sheets, and earning spots on the Big Ten All-Tournament team and CSC Academic All-District honors. She played a crucial role in key matches and penalty shootouts, demonstrating leadership and skill until her senior year, when her career was interrupted by her cancer diagnosis.
What Was Mia Hamm’s Diagnosis and Cancer Journey?
Mia Hamant was diagnosed in April 2025 with a very rare and aggressive form of stage 4 kidney cancer known as SMARCB1-deficient renal medullary carcinoma (RMC). Her initial symptoms began with shortness of breath, which led her to the emergency room. After numerous scans and tests, including a biopsy, the diagnosis was confirmed. This type of kidney cancer is extremely rare, with Mia’s case being among the very few documented.
The diagnosis profoundly impacted her life. She immediately stepped away from the University of Washington women’s soccer team to begin chemotherapy and radiation treatments. Despite the severe illness and treatment side effects, Mia remained open about her journey, sharing her experience on social media to inspire and raise awareness. Her battle with cancer not only interrupted her promising sports career but also deeply affected her personal life, as she faced the challenges of a terminal diagnosis with remarkable bravery and resilience.

Photo:Mia Hamant Instagram account
What Is Mia Hamm’s Support and Community Impact?
Mia Hamant received strong and heartfelt support from her teammates, coaches, family, and the wider community throughout her cancer battle. The University of Washington’s women’s soccer program embraced her fully, ensuring she remained an integral part of the team despite her illness. They attended her games, posted supportive signs, and helped organize fundraising events to assist with her medical expenses and raise awareness about her rare cancer.
Examples of this support include contributions from coaches and classmates, local businesses like coffee stands rallying behind her, and public displays of solidarity on social media. Mia celebrated life milestones like her birthday surrounded by her community, which helped her maintain a sense of normalcy amid the challenges of treatment. The team also dedicated their efforts in tournaments as a tribute to her resilience, such as winning the Big Ten Tournament shortly after her passing, demonstrating how deeply her fight inspired those around her.
Mia’s story has further fueled awareness of SMARCB1-deficient renal medullary carcinoma, motivating fundraising and advocacy efforts in kidney cancer research, fueled by the genuine connection and love she inspired within her community.
Scientific Overview: SMARCB1-Deficient Renal Medullary Carcinoma (RMC)
SMARCB1-deficient renal medullary carcinoma is an extremely rare and highly aggressive kidney cancer classified under “SMARCB1-deficient renal carcinomas” in the World Health Organization (WHO) Classification of Tumors of the Urinary System and Male Genital Organs, 5th Edition (2022). RMC accounts for well under 1% of all renal cell carcinomas worldwide, making it one of the rarest entities described in the WHO classification system. It is characterized by the loss of the SMARCB1 (INI1) tumor suppressor gene, a molecular hallmark that the WHO specifies as essential for diagnosis.
Although historically linked to individuals with sickle cell trait, WHO notes that SMARCB1-deficient renal carcinomas can also occur in people without hemoglobinopathies, broadening the understanding of this disease beyond its classical description. Most patients are children, adolescents, or young adults, and the disease is known for its rapid progression, early metastasis, and high mortality.
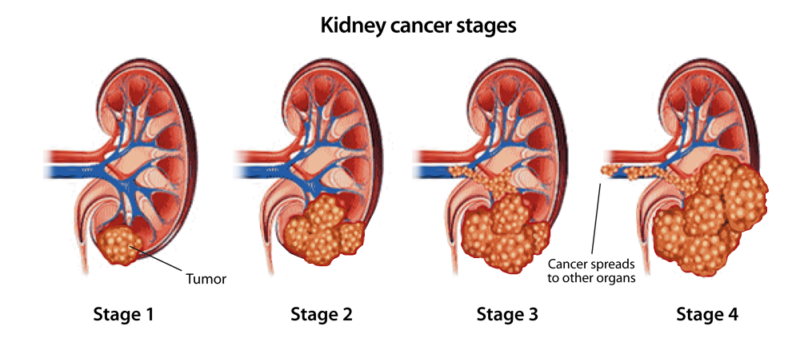
Scientific Characteristics and Tumor Biology
According to WHO and recent oncologic literature, RMC arises in the renal medulla, the innermost portion of the kidney. Microscopically, it is described as a high-grade carcinoma with infiltrative growth patterns, rhabdoid morphology, and complete loss of SMARCB1 protein expression on immunohistochemistry. The absence of SMARCB1 disrupts chromatin remodeling through the SWI/SNF pathway, resulting in uncontrolled cellular proliferation. Modern genomic studies show that this pathway alteration is a defining biological driver of this cancer.
Clinical Presentation
Patients often present at an advanced stage because the cancer progresses rapidly. The most common symptoms include flank or abdominal pain, blood in the urine, unintended weight loss, fever, and fatigue. Many young patients, like in Mia’s case, first notice symptoms such as shortness of breath, which is often due to early lung metastases, a pattern frequently documented in case series. Because RMC affects younger people who are otherwise healthy, the initial symptoms are often overlooked or misattributed.
Diagnosis
Diagnosis relies on imaging, biopsy, and molecular confirmation. CT and MRI scans typically reveal an ill-defined, infiltrative tumor in the renal medulla, often with involvement of lymph nodes. WHO guidelines emphasize that immunohistochemistry showing loss of SMARCB1/INI1 expression is essential for confirming SMARCB1-deficient carcinoma.
Pathology reports usually show a high-grade carcinoma with solid, reticular, or cribriform patterns. Because of the rarity of the disease, diagnosis often requires expert review at specialized centers.
Treatment Approaches
There is no standardized global treatment protocol, primarily because of the cancer’s rarity, but the WHO and leading oncology guidelines note that platinum-based chemotherapy is the most frequently used first-line therapy. Regimens such as cisplatin combined with gemcitabine may temporarily shrink tumors or stabilize disease. However, responses are usually short-lived because of the tumor’s aggressive nature.
Targeted therapies used in other renal cancers, such as VEGF inhibitors or standard tyrosine kinase inhibitors, generally have limited effectiveness in RMC. Immunotherapy has been attempted in several cases, but published literature reports inconsistent outcomes. Surgery is rarely curative because the cancer is typically metastatic at diagnosis, although nephrectomy may be considered in selected patients to reduce tumor burden. Radiation therapy is used mainly for symptom control, such as relief of bone pain or management of specific metastatic lesions.
Prognosis
SMARCB1-deficient renal medullary carcinoma has one of the poorest outcomes among renal malignancies described by WHO. Historical survival rates indicate a median survival of approximately 4 to 13 months from diagnosis, even with aggressive treatment. Fewer than 10% of patients survive beyond two years, according to pooled analyses published in peer-reviewed oncology journals and summarized in the WHO classification. Young age does not improve prognosis due to the tumor’s inherently aggressive biological profile.
Current Research and Future Directions
Research efforts are focused on understanding the molecular consequences of SMARCB1 loss. After WHO formally categorized these tumors within the “SMARCB1-deficient” family, scientific interest increased in therapies targeting EZH2, an enzyme that becomes overactive when SMARCB1 is lost. Early-phase studies of EZH2 inhibitors have shown potential in stabilizing disease in some SMARCB1-deficient tumors, though data in RMC remains limited.
Global cancer registries, molecular tumor banks, and rare-cancer advocacy groups are now working to collect more cases, as increasing awareness is essential for developing better treatments.
You Can Also Read In Memory of TikToker Bailey Hutchins and Her Battle Against Colorectal Cancer by OncoDaily

Written by Aharon Tsaturyan, MD, Written by Aharon Tsaturyan, MD, Editor at OncoDaily Intelligence Unit


