Itaconate is an immunomodulatory metabolite traditionally associated with activated macrophages, where it plays roles in inflammation, host defense, and metabolic regulation. Its production is catalyzed by the enzyme cis-aconitate decarboxylase 1 (ACOD1), also known as IRG1.
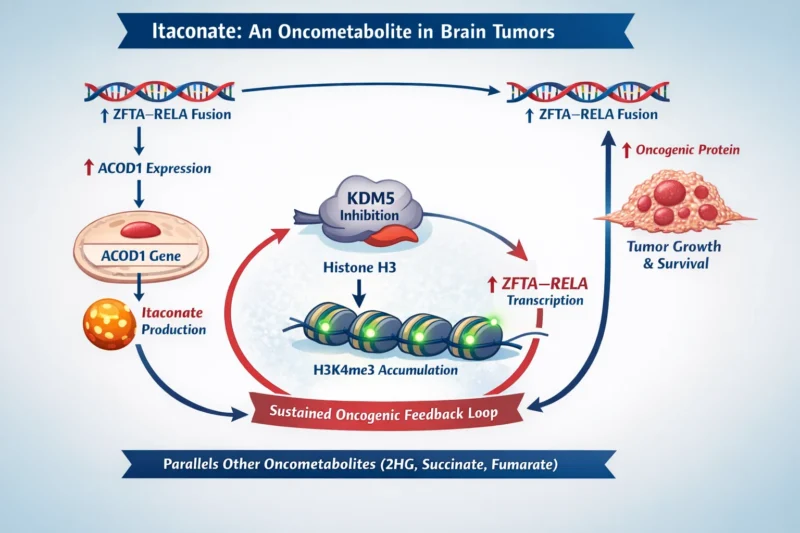
Recent research has demonstrated that ZFTA–RELA–positive ependymoma cells aberrantly express ACOD1 and produce substantial amounts of itaconate. Metabolomic profiling revealed that itaconate is among the most upregulated metabolites in cells expressing the ZFTA–RELA fusion compared with non-fusion controls.
This finding is particularly significant because it establishes that tumor cells not just immune cells can generate itaconate, suggesting a tumor-intrinsic role beyond immune modulation.
Critically, ACOD1 expression itself is driven by the ZFTA–RELA fusion, creating a metabolic program directly controlled by the oncogenic driver.
Itaconate Functions as an Oncometabolite Through Epigenetic Regulation
The most striking discovery is that itaconate sustains tumor growth through epigenetic mechanisms.
Itaconate inhibits KDM5, a histone demethylase responsible for removing trimethyl groups from histone H3 lysine 4 (H3K4me3), a chromatin mark associated with active transcription. Inhibition of KDM5 results in accumulation of H3K4me3, particularly at regulatory regions of the ZFTA gene.
This epigenetic modification enhances transcription of the ZFTA–RELA fusion itself, creating a feed-forward loop:
- ZFTA–RELA induces ACOD1 expression
- ACOD1 produces itaconate
- Itaconate inhibits KDM5
- Increased H3K4me3 enhances ZFTA–RELA transcription
- Elevated ZFTA–RELA further reinforces ACOD1 expression
This feedback loop ensures sustained expression of the oncogenic fusion protein, which is essential for tumor survival and proliferation.
This mechanism parallels other well-known oncometabolites, such as 2-hydroxyglutarate, succinate, and fumarate, which alter epigenetic regulation in cancer. These findings position itaconate as a previously unrecognized oncometabolite in brain tumors.
Metabolic Reprogramming: Glutamine Fuels Itaconate Production
Further investigation revealed that ZFTA–RELA–positive tumors undergo metabolic reprogramming to support itaconate synthesis.
Glutamine, a key nutrient in cancer metabolism, serves as the primary carbon source for itaconate production through its integration into the tricarboxylic acid (TCA) cycle.
These tumors exhibit:
- Increased glutamine uptake
- Elevated glutaminase expression
- Enhanced glutamine flux into itaconate biosynthesis
This glutamine dependence represents a metabolic vulnerability.
Mechanistically, the ZFTA–RELA fusion suppresses PTEN, a tumor suppressor that normally restrains PI3K–AKT–mTOR signaling. Loss of PTEN activity enhances PI3K–mTOR signaling, which drives glutamine metabolism and supports itaconate synthesis.
Thus, ZFTA–RELA orchestrates a coordinated metabolic and epigenetic program to sustain its own expression.
Therapeutic Targeting of Itaconate Metabolism Shows Promising Results
Importantly, disruption of this pathway produces significant therapeutic effects in preclinical models.
Several strategies demonstrated efficacy:
ACOD1 inhibition
Genetic deletion or pharmacologic inhibition of ACOD1:
- Reduced itaconate production
- Decreased ZFTA–RELA expression
- Suppressed tumor growth
- Prolonged survival in animal models
These results confirm that tumor cells depend on ACOD1-mediated itaconate production.
Targeting glutamine metabolism
Inhibitors of glutamine utilization, including CNS-penetrant agents, reduced tumor growth and decreased ZFTA–RELA levels.
Because glutamine is required to generate itaconate, blocking glutamine metabolism disrupts the entire oncogenic feedback loop.
PI3K–mTOR pathway inhibition
Inhibition of PI3K–mTOR signaling restored PTEN-regulated metabolic control and reduced glutamine utilization, further impairing tumor growth.
Combination therapies targeting multiple nodes of this metabolic network showed the strongest effects.
Prevention of tumor metastasis
Notably, targeting itaconate production or combining metabolic inhibitors prevented spinal metastasis in animal models an important clinical complication associated with poor outcomes.
A New Paradigm: Metabolism Sustains Oncogenic Fusion Proteins
These findings fundamentally expand our understanding of how oncogenic fusion proteins are maintained in cancer.
Rather than acting solely through transcriptional or signaling pathways, ZFTA–RELA relies on a metabolite-driven epigenetic feedback loop to sustain its own expression.
This represents a paradigm shift, highlighting how tumor metabolism and epigenetics cooperate to reinforce oncogenic drivers.
Translational and Clinical Implications
This work has important implications for future therapeutic development in neuro-oncology.
Key translational opportunities include:
- Targeting ACOD1 directly
- Inhibiting glutamine metabolism
- Combining metabolic inhibitors with PI3K–mTOR inhibitors
- Developing therapies that disrupt oncometabolite-mediated epigenetic regulation
Because these strategies reduce expression of the oncogenic driver itself, they offer a fundamentally different therapeutic approach compared with conventional cytotoxic or signaling-based therapies.
Moreover, these findings establish itaconate as a bona fide oncometabolite and highlight metabolic-epigenetic dependencies as promising targets in fusion-driven cancers.
Targeting Itaconate Opens a New Therapeutic Avenue in Ependymoma
ZFTA–RELA–positive ependymomas exploit a macrophage-like metabolic pathway to produce itaconate, which epigenetically sustains the expression of the oncogenic fusion protein.
This discovery reveals a previously unrecognized metabolic dependency and identifies multiple actionable therapeutic targets.
By disrupting the metabolic-epigenetic feedback loop centered on itaconate, researchers have uncovered a promising strategy to suppress tumor growth and metastasis.
These findings not only offer hope for improved therapies in ependymoma but also underscore the broader importance of oncometabolites as drivers of cancer biology and therapeutic vulnerability.
How Bacteria May Promote Breast Cancer: The Emerging Role of SMOX
Written by Nare Hovhannisyan, MD



