Senthil Kumar, a Medical Oncologist in Chennai, Tamil Nadu, India, shared a post on X:
“First-Line Treatment in Recurrent / Metastatic Cervical Cancer
KEYNOTE-826: Pembrolizumab + Chemotherapy ± Bevacizumab
Study Design: Phase III trial evaluating pembrolizumab combined with chemotherapy (± bevacizumab) for advanced cervical cancer.
Patients: Persistent, recurrent, or metastatic cervical cancer not amenable to curative treatment.
Key Outcomes:
PD-L1 CPS ≥1 Population:
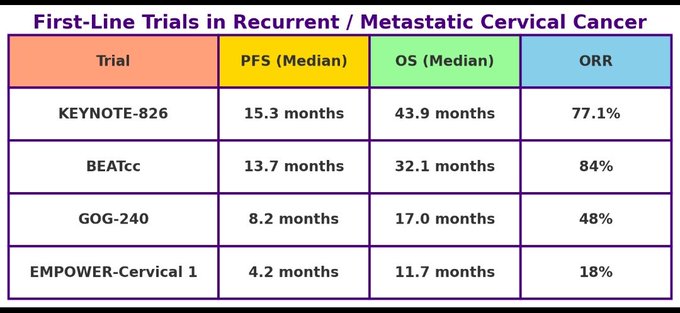
- Progression-Free Survival (PFS): With Bevacizumab: 15.3 months (pembrolizumab + chemo + bevacizumab) vs. 10.3 months (chemo + bev) (HR = 0.56). Without Bevacizumab: 7 months (pembrolizumab + chemo) vs. 6 months (placebo + chemo) (HR = 0.69).
- Overall Survival (OS): With Bevacizumab: 43.9 months (pembrolizumab + chemo + bevacizumab) vs. 23.0 months ( chemo + bev) (HR = 0.60). Without Bevacizumab: 17.5 months (pembrolizumab + chemo) vs. 11.9 months ( chemo ). All Comers Population:
- Progression-Free Survival (PFS): With Bevacizumab: 15.2 months (pembrolizumab + chemo + bevacizumab) vs. 10.2 months (chemo + bev). Without Bevacizumab: 6.3 months (pembrolizumab+ chemo) vs. 6.2 months (chemo).
- Overall Survival (OS): With Bevacizumab: 37.6 months (pembrolizumab + chemo + bevacizumab) vs. 22.5 months (chemo + bev ). Without Bevacizumab: 17.1 months (pembrolizumab + chemo) vs. 12.6 months (chemo).
BEATcc Trial: Atezolizumab + Bevacizumab + Chemotherapy
Study Design: Open-label Phase III trial evaluating atezolizumab combined with bevacizumab and chemotherapy for metastatic or recurrent cervical cancer.
Key Outcomes:
- Progression-Free Survival (PFS): 13.7 months (atezolizumab arm) vs. 10.4 months (control arm) (HR = 0.62).
- Overall Survival (OS): 32.1 months (atezolizumab arm) vs. 22.8 months (control arm) (HR = 0.68).
- Objective Response Rate (ORR): 84% (atezolizumab arm) vs. 72% (control arm).
GOG-240: Bevacizumab + Chemotherapy
Study Design: Phase III trial establishing bevacizumab with chemotherapy as the standard for recurrent/metastatic cervical cancer.
Key Outcomes:
- Progression-Free Survival (PFS): 8.2 months (bevacizumab arm) vs. 5.9 months (chemo alone).
- Overall Survival (OS): 17.0 months (bevacizumab arm) vs. 13.3 months (chemo alone).
- Objective Response Rate (ORR): 48% (bevacizumab) vs. 36% (without).
EMPOWER-Cervical 1: Cemiplimab
Study Design: Phase III trial investigating cemiplimab monotherapy a PD-1 inhibitor, for platinum-refractory recurrent/metastatic cervical cancer.
Key Outcomes:
- Progression-Free Survival (PFS): 4.2 months (cemiplimab) vs. 3.0 months (chemotherapy).
- Overall Survival (OS): 11.7 months (cemiplimab) vs. 8.5 months (chemo) (HR = 0.66).
Key Insights:
- Quadruplet Regimen: Combining immunotherapy, a chemotherapy doublet, and bevacizumab provides outstanding PFS and OS benefits across PD-L1 subsets.
- For Bevacizumab-Ineligible Patients: Pembrolizumab with a chemotherapy doublet remains a strong option.
- Affordability Considerations: Bevacizumab with a chemotherapy doublet is a cost-effective alternative.“
More posts featuring Senthil Kumar.