Bispecific Antibody M701 Offers a Novel Treatment Option for Malignant Ascites
Malignant ascites is a severe manifestation of advanced cancers, frequently seen in ovarian, gastric, colorectal, lung, and breast malignancies, as well as in cases of cancer of unknown primary. It results from tumor-induced mechanisms such as increased vascular permeability, lymphatic obstruction, and an immunosuppressive tumor microenvironment. This leads to the pathological accumulation of fluid in the peritoneal cavity, significantly impairing patient quality of life and reducing survival outcomes.
The clinical burden of malignant ascites includes symptoms like abdominal swelling, pain, dyspnea, fatigue, nausea, and nutritional deficiencies, which profoundly affect daily functioning and mental well-being. Prognosis is typically poor, with a median overall survival ranging from 1 to 6 months. Current management approaches, such as paracentesis and indwelling peritoneal catheters, offer symptomatic relief but are temporary and often accompanied by complications like infections, electrolyte imbalances, and the need for repeated procedures. These challenges highlight the critical need for innovative therapies targeting the underlying pathophysiology of malignant ascites.
Exciting developments in this field were presented at the 2024 European Society for Medical Oncology Asia Congress (ESMO Asia). Professor Rongrui Liu from the Chinese PLA General Hospital discussed the findings from a Phase II clinical trial (NCT06266091, Abstract No. 61O) evaluating M701, a bispecific antibody developed by Wuhan YZY Biopharma. Representing the study’s principal investigator, Professor Jianmin Xu, Professor Liu highlighted the promising potential of M701 in transforming malignant ascites management by addressing its root causes, paving the way for improved patient outcomes. Moreover, the Oncology Frontier spoke with Prof. Liu to gain deeper insights into the study.

What is M701 and How Does It Work?
M701 is an advanced bispecific antibody that marks a significant advancement in the management of malignant ascites. It targets EpCAM (epithelial cell adhesion molecule), a protein overexpressed on epithelial tumor cells, and CD3, a molecule on T cells. By linking tumor cells and T cells, M701 enhances the immune system’s ability to recognize and destroy malignant cells within the peritoneal and pleural cavities.
M701 operates through a multifaceted mechanism. Its Fab region binds to EpCAM for precise targeting of tumor cells, while the scFv region engages CD3 on T cells, activating them to attack EpCAM-positive cells. The humanized Fc region amplifies immune responses by recruiting natural killer cells and activating the complement system, driving tumor cell destruction through antibody-dependent and complement-dependent cytotoxicity (ADCC and CDC). This approach reduces tumor proliferation, alleviates fluid accumulation, and improves patient quality of life.
It is worth noting the historical and renewed importance of EpCAM-targeting therapies. Approved in Europe in 2009, catumaxomab—a bispecific antibody targeting EpCAM and CD3—was later withdrawn from the market for commercial reasons. However, in October 2024, it received a positive opinion from the Committee for Medicinal Products for Human Use (CHMP), reflecting a renewed recognition of its value in the market.
Developed through YZY Biopharma’s YBODY® platform, M701 exemplifies cutting-edge bispecific antibody engineering. This platform employs advanced techniques to ensure high specificity and low immune cell affinity, minimizing risks of unintended immune activation while maintaining therapeutic potency.
By directly addressing the root causes of malignant ascites, M701 offers a promising treatment option. Its ability to enhance immune engagement while maintaining a favorable safety profile positions it to potentially redefine the standard of care for malignant ascites and related malignancy-associated complications.
Scientific Data Presented at ESMO Asia 2024
At the ESMO Asia Congress 2024, Professor Rongrui Liu presented groundbreaking findings from a Phase II randomized, open-label, controlled, multicenter trial (NCT06266091) evaluating the efficacy and safety of M701, an anti-EpCAM x anti-CD3 bispecific antibody, for the treatment of malignant ascites caused by advanced epithelial cancers. The results revealed the transformative potential of M701 in addressing this challenging condition, particularly compared to traditional symptom management strategies like paracentesis.
The trial enrolled 84 patients with advanced epithelial tumors, including gastric, ovarian, and colorectal cancers, all of whom exhibited moderate to severe ascites despite prior second-line treatments. Patients were randomized into two groups: one receiving intraperitoneal M701 infusions alongside systemic therapy (n=43) and the other undergoing paracentesis with systemic therapy (n=41). The study’s primary endpoint was puncture-free survival (PuFS), with secondary endpoints including progression-free survival (PFS), overall survival (OS), and safety.
M701 was administered intraperitoneally to directly target tumor cells in the peritoneal cavity, leveraging its unique ability to bridge EpCAM-positive tumor cells with T cells to enhance immune-mediated destruction. This approach aimed to provide long-term symptom relief by addressing the underlying cause of fluid accumulation.
Key Findings
The study demonstrated clear superiority of M701 over paracentesis in terms of efficacy, with remarkable improvements in both PuFS and OS, particularly in specific subpopulations:
- Puncture-Free Survival (PuFS):
- In the full analysis set (FAS), median PuFS was 75 days for the M701 group compared to 23 days for the control group (HR 0.40; 95% CI: 0.21–0.76; p=0.0085).
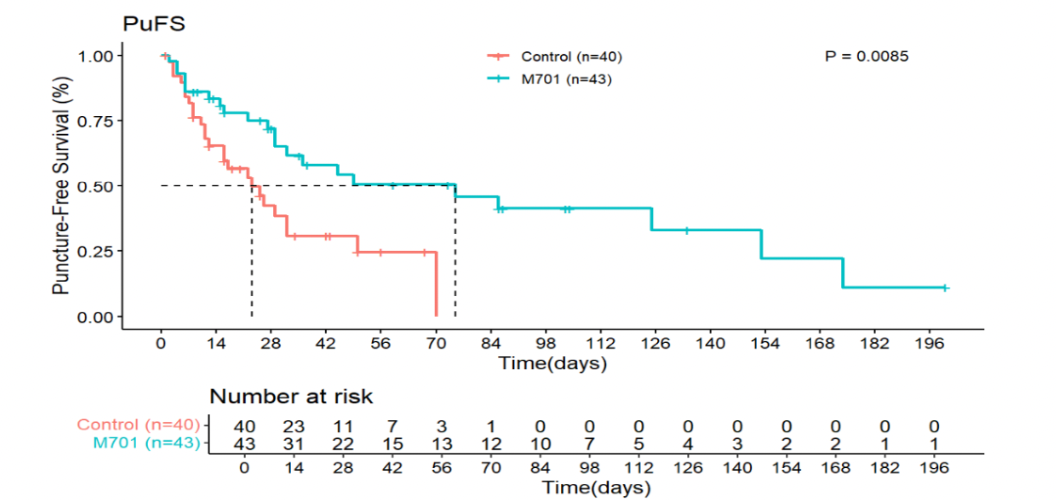
-
- Among patients with ≥13% relative lymphocyte count (RLC), median PuFS improved further to 125 days for M701 versus 22 days for controls (HR 0.22; 95% CI: 0.10–0.51; p=0.0001).
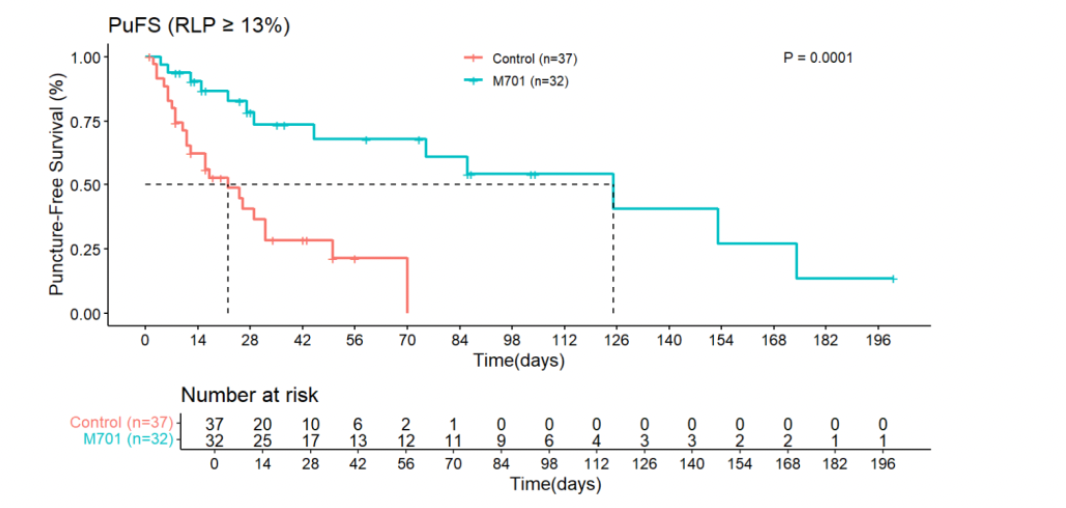
- Overall Survival (OS):
- In the FAS population, median OS was 111 days for M701 compared to 86 days for controls (HR 0.65; 95% CI: 0.39–1.09; p=0.102).
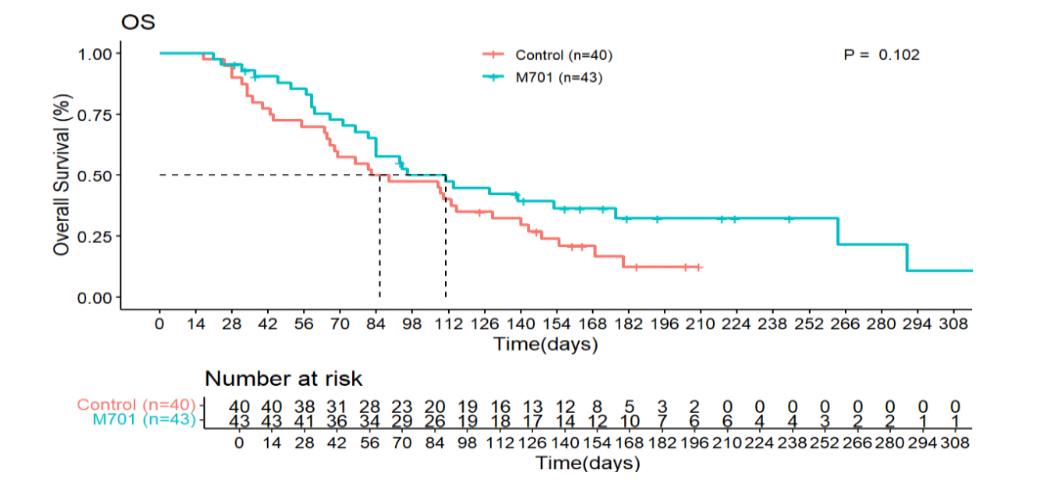
-
- In the ≥13% RLC subpopulation, median OS reached 139 days for M701 versus 82 days for controls (HR 0.51; 95% CI: 0.28–0.92; p=0.026).
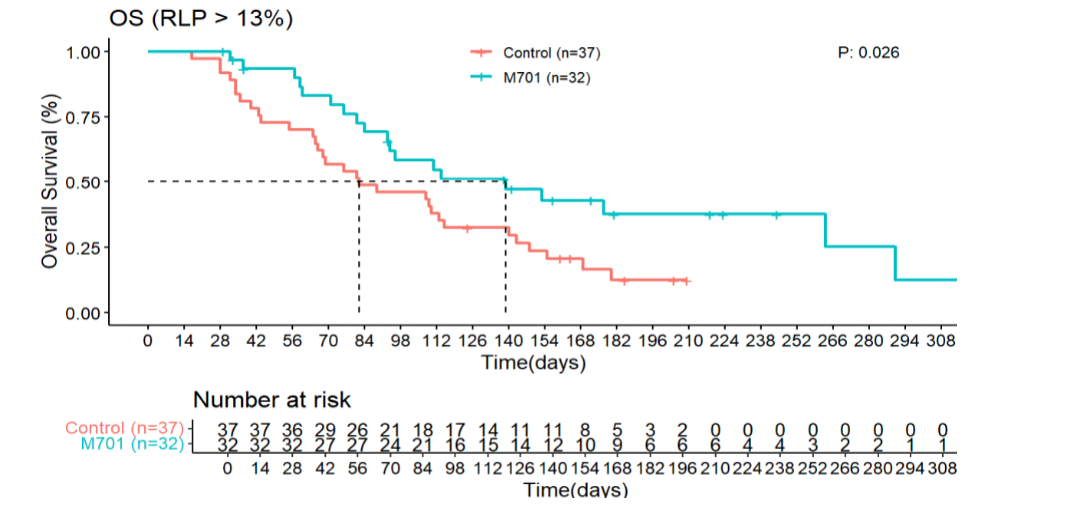
- Safety Profile:
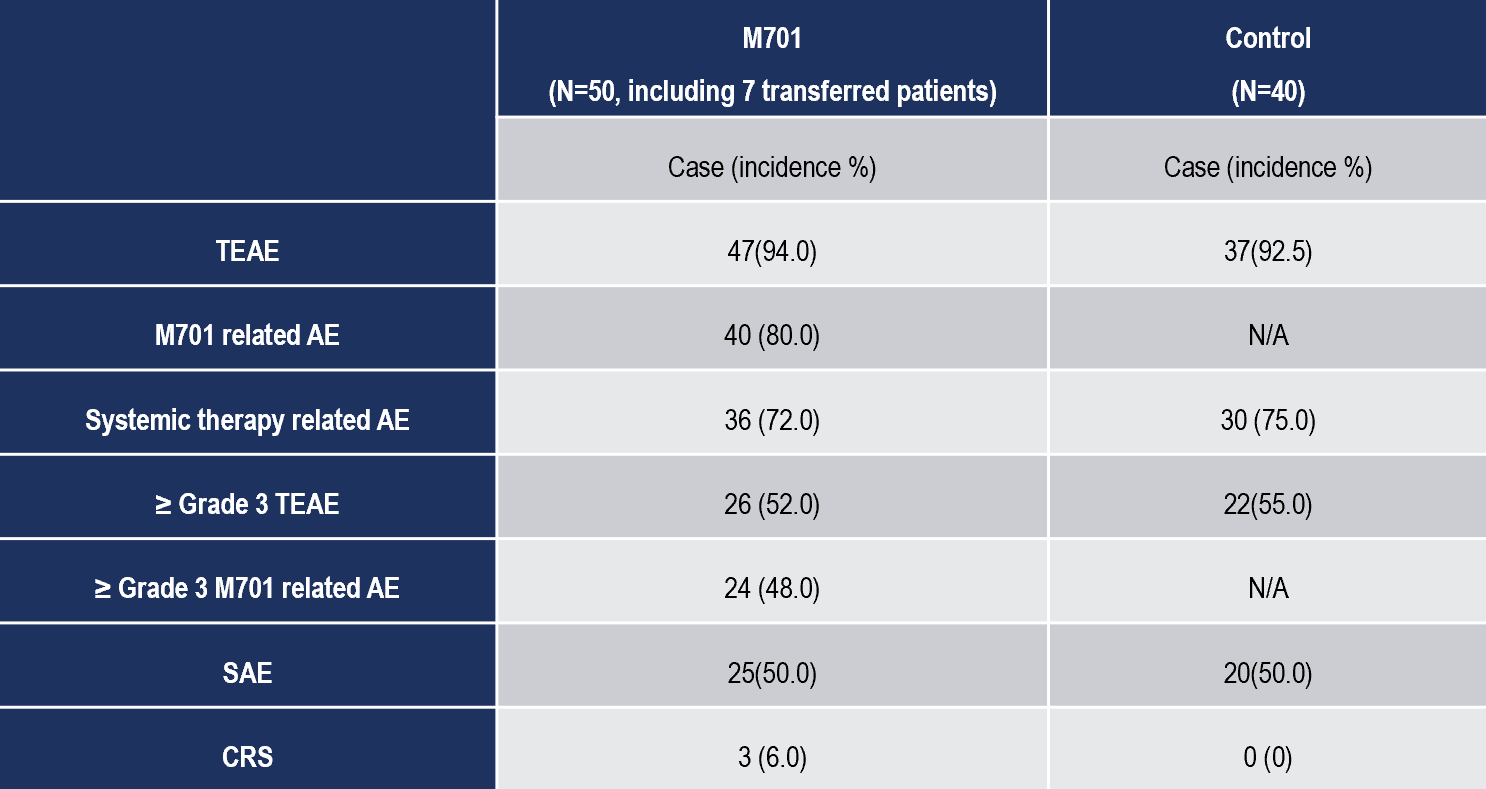
- M701 demonstrated an acceptable safety profile, with no unexpected adverse events. Treatment-related adverse events (TRAEs) occurred in 80% of the M701 group but were generally manageable. Notably, the incidence of cytokine release syndrome (CRS) was low (6%) and did not result in treatment discontinuation.
- Grade ≥3 adverse events were observed in 48% of patients in the M701 group, comparable to the control group’s 55%, underscoring the tolerability of the new therapy.
Safety overview
The Phase II trial subgroup analysis underscored the importance of Relative Lymphocyte Count as a predictive biomarker for M701’s efficacy. Patients with higher RLC levels, indicative of better-preserved immune function, experienced the most significant benefits in both Puncture-Free survival and overall survival. These findings highlight the potential for personalized treatment strategies, where patient selection based on immune biomarkers could optimize therapeutic outcomes and maximize the benefits of M701.
Clinical Perspectives on M701
Clinical experts have underscored the transformative potential of M701 in managing malignant ascites and pleural effusions. By targeting EpCAM-positive tumor cells directly within the peritoneal and pleural cavities, M701 offers a more effective and durable solution compared to traditional symptom management approaches. This targeted approach not only reduces fluid accumulation and recurrence but also enhances overall survival outcomes, improving the quality of life for patients. Administering M701 via intraperitoneal infusion requires meticulous procedural planning and patient education. Clinicians must address potential challenges such as catheter-related complications, leakage, and infection risks. Providing patients with comprehensive guidance on catheter care, dressing changes, and post-discharge monitoring is critical to minimizing complications and ensuring treatment success.
Comparative analyses from clinical observations suggest that M701 offers superior local control of malignant ascites compared to routine intraperitoneal chemotherapy, which often provides only temporary relief. Patients treated with M701 experienced prolonged symptom control and fewer adverse events, reaffirming its role as a next-generation therapy for malignant ascites management.

Expert Opinions
Leading clinical experts have emphasized the transformative potential of M701 in addressing malignant ascites.

“This is the best drug I have encountered in my over 20 years of medical practice for treating malignant ascites caused by gastrointestinal tumors. Many patients, after using it, no longer require repeated paracentesis for a long period, significantly improving their quality of life”: Jianmin Xu

“Many patients with advanced cancer develop ascites, which significantly affects their quality of life, causing symptoms such as abdominal distension, loss of appetite, and even breathing difficulties. Clinically, there is a notable lack of effective treatments, especially after second-line therapy, as patients receiving later-line treatments often show poor sensitivity to chemotherapy. M701 offers an effective solution to address treatment resistance in ascites management. Clinical observations have shown that M701 not only improves Puncture-Free Survival (PuFS) but also enhances patients’ quality of life and overall survival time”: Rongbo Lin
See Dr. Rongbo Lin’s interview on M701 at ASCO2024 by OncoDaily
This article is a collaborative post developed by the OncoDaily editorial team, utilizing data provided by YZY Biopharma: SPONSORED


