At ASCO 2025, Dr. Alessandro Leonetti and colleagues from the Italian GOIRC consortium presented final results from the ALNEO study, a multicenter, phase II trial evaluating neoadjuvant and adjuvant alectinib in patients with potentially resectable stage III ALK-positive non-small cell lung cancer (NSCLC). This is the first prospective trialto assess the role of alectinib in the perioperative setting for locally advanced ALK+ NSCLC, a population with limited data and significant therapeutic need.
What Is the ALNEO Trial?
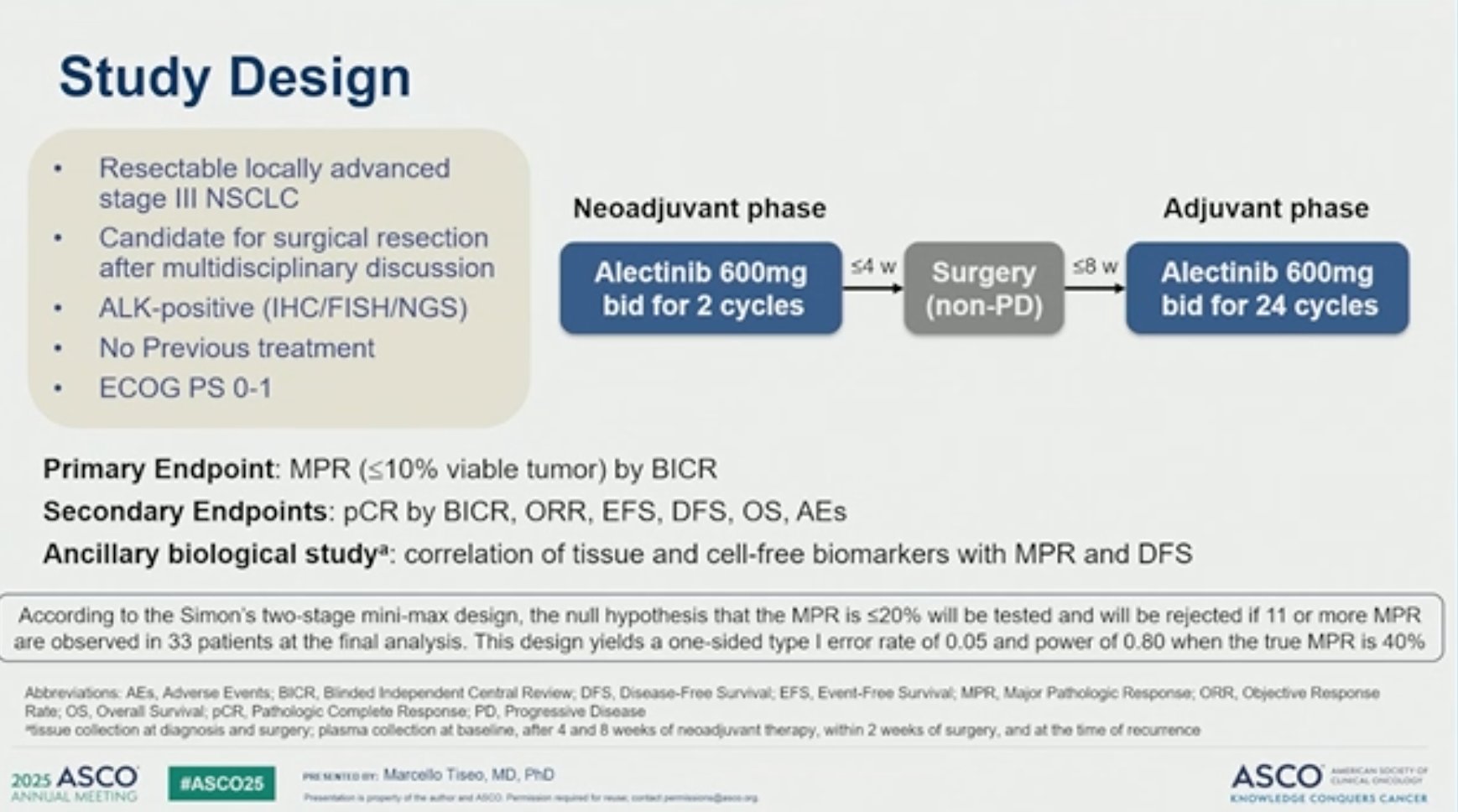
The ALNEO study (EUDRACT 2020-003432-25) is an open-label, single-arm, multicenter phase II trial conducted across 20 oncology centers in Italy. The trial enrolled treatment-naïve patients with stage IIIA or IIIB ALK-positive NSCLC who were considered potentially resectable, with ECOG performance status ≤1.
-
Neoadjuvant treatment: Alectinib for 2 cycles (8 weeks)
-
Surgical resection, followed by:
-
Adjuvant alectinib for up to 24 cycles (96 weeks)
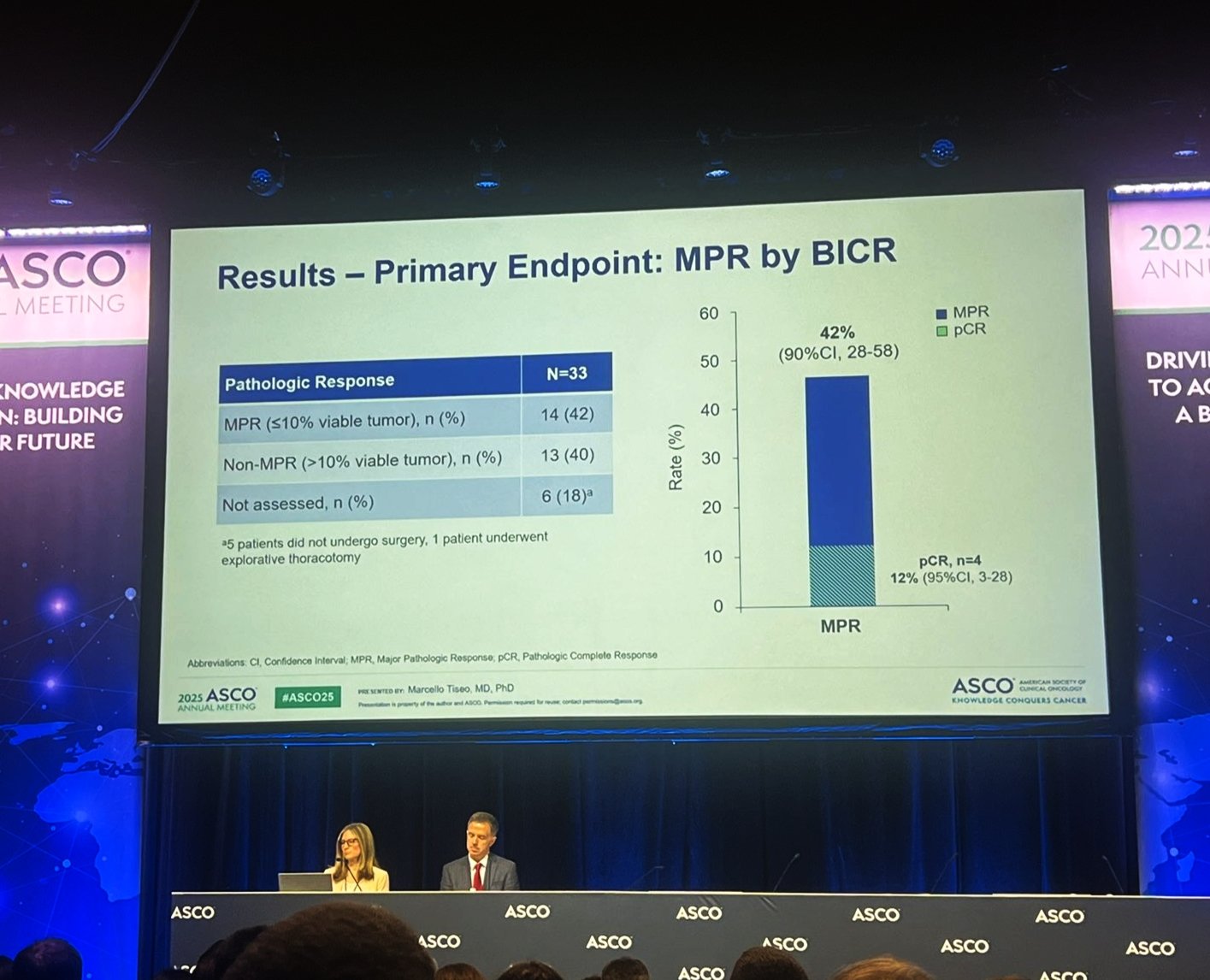
The primary endpoint was major pathological response (MPR) assessed by Blinded Independent Central Review (BICR). Secondary endpoints included pathological complete response (pCR), objective response (OR), event-free survival (EFS), disease-free survival (DFS), overall survival (OS), and safety.
A Simon’s two-stage design was employed to assess efficacy, requiring 33 patients for full enrollment.
 Key Findings
Key Findings
Between May 2021 and July 2024, the ALNEO trial enrolled 33 patients with potentially resectable stage III ALK-positive non-small cell lung cancer (NSCLC) across 20 centers in Italy. The median age of participants was 62 years(interquartile range: 49–74), with a predominance of female patients (70%) and never-smokers (52%)—a clinical profile commonly associated with ALK-positive lung cancer.
Staging based on the AJCC 8th edition revealed that 64% of patients (21 individuals) had stage IIIA disease, while the remaining 36% (12 patients) had stage IIIB. The most frequently observed substage was T3N2 (24%), followed by T1aN2, T2aN2, T4N0, and T4N2—each accounting for 12% of the study population.
All 33 patients successfully completed neoadjuvant therapy with alectinib, and 28 patients (85%) proceeded to surgery. Among those who underwent resection:
-
21 patients (64%) received a lobectomy
-
3 patients (9%) underwent pneumonectomy
-
4 patients (12%) had other types of surgery
Importantly, 24 of the 28 operated patients (86%) achieved an R0 resection, meaning there was no residual microscopic tumor at the surgical margins.
Efficacy Outcomes
Treatment with neoadjuvant alectinib led to a major pathological response (MPR)—defined as ≤10% viable tumor cells in the resected specimen—in 15 patients (46%), as assessed by Blinded Independent Central Review (BICR). The 90% confidence interval for this response ranged from 31% to 61%. Additionally, 4 patients (12%) achieved a complete pathological response (pCR), with no viable tumor cells detected in the surgical specimen (95% CI: 3%–28%).
Radiographic evaluations revealed an objective response (OR) in 22 patients (67%), supporting strong preoperative tumor shrinkage with targeted therapy.
Following surgery, 26 patients (79%) began adjuvant treatment with alectinib, typically initiated within 5.1 weekspost-surgery (IQR: 3.6–6.0). At a median follow-up of 15.2 months (IQR: 6.8–27.8), 94% of patients were alive, and 5 patients (19%) had completed the full 2-year course of adjuvant therapy.
So far, median event-free survival (EFS) and overall survival (OS) have not yet been reached, reflecting encouraging durability of disease control. Only 6 patients (18%) experienced disease progression or recurrence during the study period.
Safety and Tolerability
Treatment with alectinib was well tolerated in both the neoadjuvant and adjuvant settings. During the preoperative phase, grade 3 or higher adverse events (AEs) were observed in 3 patients (9%), while during adjuvant therapy, 2 patients (8%) experienced grade ≥3 AEs.
These low toxicity rates are consistent with the known safety profile of alectinib in the metastatic setting and support its favorable risk-benefit ratio in the perioperative management of ALK-positive NSCLC.
What This Means for Patients
The ALNEO trial met its primary endpoint, demonstrating a 46% major pathological response rate and favorable surgical outcomes, with high R0 resection rates. These findings suggest that alectinib is both active and feasible as a perioperative therapy in patients with resectable stage III ALK-positive NSCLC, offering an alternative to cytotoxic chemotherapy in this molecularly defined population.
Importantly, this is the first trial to evaluate a targeted neoadjuvant approach in ALK+ NSCLC, and the strong response rates and surgical outcomes provide a compelling case for further exploration in larger, randomized trials.
What People Are Saying About the ALNEO Trial?
Dr. Jarushka Naidoo, Professor of Medical Oncology and thoracic cancer expert, immunotherapy researcher, and Johns Hopkins adjunct faculty, shared on X.
“ASCO25 Lung mini orals
Phase II ALNEO trial: Neoadjuvant alectinib for two cycles followed by surgery and up to 24 months of adjuvant alectinib: 33 patients enrolled, 86% achieved R0 resection, Major pathologic response (MPR) in 42%, complete pathologic response (pCR) in 12%, Minimal toxicity observed in both neoadjuvant and adjuvant phases. Results exceeded expectations compared to the EGFR space, though limited by small sample size.”