
What Is Stereotactic Ablative Radiotherapy (SABR) and How It Works? Pros and Cons
SABR is an advanced radiation therapy that delivers a highly concentrated radiation dose directly to tumors, minimizing damage to surrounding healthy tissue. Utilizing advanced imaging and delivery, SABR allows for fewer, more intense treatment sessions than traditional radiation. This method is effective for various cancers, especially early-stage or limited metastatic disease. While SABR offers benefits like shorter treatment times and reduced side effects, its suitability for each patient must be carefully considered.
What Is Stereotactic Ablative Radiotherapy (SABR)?
Stereotactic ablative radiotherapy (SABR), also referred to as stereotactic body radiation therapy (SBRT), is a sophisticated radiation therapy technique designed to deliver a highly concentrated dose of radiation directly to a tumor. This method employs advanced imaging technologies, such as CT scans, to precisely locate and target the tumor, enabling the delivery of intense radiation while significantly limiting exposure to adjacent healthy tissues. By utilizing millimeter-scale accuracy and specialized immobilization devices, SABR ensures that the radiation is delivered with exceptional precision, thereby maximizing tumor destruction and minimizing potential side effects. This approach enables the delivery of higher doses in fewer sessions compared to conventional radiation therapy, improving treatment efficiency and patient comfort.
How Does SABR Work?
SABR employs advanced imaging, such as CT scans, to precisely locate and map tumors. Multiple radiation beams, shaped and directed from various angles, converge on the tumor, delivering a high dose of radiation. Real-time imaging and immobilization devices ensure accurate delivery, minimizing damage to surrounding healthy tissues. This precise approach allows for effective tumor ablation in fewer treatment sessions than traditional radiation therapy.
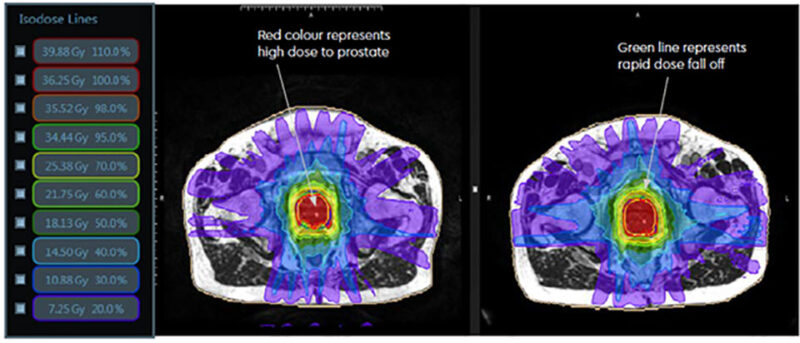
source: www.eastmidlandsurology.com
What Are the Main Types of Stereotactic Ablative Radiotherapy?
SABR uses diverse techniques for precise tumor targeting, optimizing outcomes. These methods deliver high-dose radiation with exceptional accuracy, limiting damage to healthy tissue. Technique selection depends on tumor location, size, and proximity to critical organs. Technologies like Linac, CyberKnife, TomoTherapy, and Gamma Knife are employed, each with unique radiation delivery capabilities. Immobilization and real-time imaging ensure millimeter accuracy. This adaptability allows SABR to treat various cancers, showcasing its versatility.
Stereotactic Body Radiation Therapy (SBRT)
Stereotactic body radiation therapy (SBRT), or SABR, delivers high-dose, precise radiation to tumors outside the brain. Advanced imaging and immobilization ensure millimeter accuracy, minimizing healthy tissue damage. This allows for fewer, high-dose treatments (1-5 sessions). SBRT treats various cancers, offering a non-invasive alternative to surgery for suitable patients by minimizing side effects.
Stereotactic Radiosurgery (SRS)
Stereotactic radiosurgery (SRS) is a highly precise radiation technique used to treat brain tumors and other intracranial conditions. It delivers a single, high dose of radiation directly to the target, minimizing exposure to surrounding healthy brain tissue. Advanced imaging, such as MRI and CT scans, allows for detailed treatment planning, ensuring accurate tumor targeting. Specialized equipment, like Gamma Knife or linear accelerators, delivers multiple radiation beams that converge on the tumor. Immobilization devices maintain patient stability during the procedure. SRS is effective for treating various conditions, including benign and malignant tumors, AVMs, and trigeminal neuralgia, offering precise tumor control with reduced risk of side effects.
BMath et al. (2022), published in Lancet Oncology, found that for small-cell lung cancer (SCLC) patients with intracranial metastatic disease (IMD), stereotactic radiosurgery (SRS) showed equitable or improved survival outcomes compared to whole brain radiotherapy (WBRT). SRS resulted in longer overall survival (HR 0.85) than WBRT with or without SRS boost. Median overall survival with SRS was 8.99 months.
CyberKnife Radiosurgery
CyberKnife radiosurgery employs robotic technology to deliver highly precise radiation to tumors, even those that move with breathing, like lung and spine tumors. Unlike traditional radiosurgery, CyberKnife’s robotic arm allows for flexible radiation delivery from multiple angles. Advanced imaging systems track the tumor’s movement in real-time, enabling the robotic arm to adjust the radiation beam accordingly. This dynamic tracking and adjustment ensure that the radiation remains accurately targeted, minimizing damage to surrounding healthy tissue. This capability is particularly beneficial for treating tumors in areas prone to movement, where precision is paramount. CyberKnife’s ability to adapt to tumor motion enhances treatment effectiveness and reduces potential side effects.
Acker et al. (2020), published in BMC Radiation Oncology, found CyberKnife Stereotactic Radiosurgery (CK-SRS) safe and effective for brain metastases in elderly patients (≥65 years). Analyzing 97 patients, they reported 79%, 55%, and 23% overall survival at 3, 6, and 12 months, respectively, and high local tumor control rates (89% at 12 months). Older age and female sex were associated with increased local progression.
Gamma Knife Radiosurgery
Gamma Knife radiosurgery is a specialized technique primarily used for treating brain tumors and neurological conditions. It delivers highly focused gamma radiation to small, localized lesions with sub-millimeter accuracy. This precision is achieved by using multiple cobalt-60 sources that converge on the target, creating a high dose of radiation while minimizing exposure to surrounding healthy brain tissue. A stereotactic frame is used to immobilize the patient’s head, ensuring accurate targeting during the procedure. Gamma Knife is particularly effective for treating benign and malignant brain tumors, arteriovenous malformations (AVMs), and trigeminal neuralgia. Its exceptional precision allows for effective tumor control and symptom relief with minimal risk of side effects, making it a valuable option for patients with complex intracranial conditions.
Sadik et al. (2018), published in the Journal of Neuro-Oncology, found Gamma Knife Radiosurgery (GKRS) safe and effective as salvage treatment for recurrent gliomas. In 92 patients, tumor control was 37% overall (50% for low-grade, 27% for high-grade). Median progression-free survival (PFS) was 10.5 months overall (50.1 for low-grade, 5.7 for high-grade), and median overall survival (OS) was 34.4 months overall (86.6 for low-grade, 12.8 for high-grade).
Proton-Based Stereotactic Radiotherapy
Proton-based stereotactic radiotherapy utilizes proton beams, rather than X-rays, to deliver radiation to tumors. A key advantage of proton therapy is its ability to precisely control the depth at which the radiation deposits its energy, known as the Bragg peak. This allows for the delivery of a high dose of radiation directly to the tumor while significantly minimizing radiation exposure to surrounding healthy tissues. This characteristic is particularly beneficial when treating tumors located near critical organs or in sensitive areas. By reducing the dose to healthy tissues, proton therapy can decrease the risk of side effects and long-term complications. In stereotactic radiotherapy, proton therapy enhances precision and minimizes collateral damage, offering an advanced treatment option for carefully selected patients.
Hoppe et al. (2010), published in Radiotherapy and Oncology, compared photon-based (xSBRT) and proton-based (pSBRT) stereotactic body radiotherapy for early-stage NSCLC. 1 They found pSBRT significantly reduced radiation doses to normal lung tissue (V5, V10, V20, V40) and critical organs like the heart and esophagus compared to xSBRT. 2 While pSBRT showed dosimetric improvements, the clinical significance remained undetermined.
Adaptive SABR
Adaptive SABR represents an emerging frontier in radiation therapy, leveraging real-time imaging and AI-driven adjustments to enhance treatment accuracy. This approach addresses the challenges posed by tumor movement and changes in patient anatomy during treatment. Real-time imaging, such as on-board CT or MRI, allows for continuous monitoring of the tumor’s position and shape. AI algorithms analyze these images, automatically adjusting the radiation beam’s shape and intensity to ensure precise targeting. This adaptability minimizes the risk of delivering radiation to healthy tissues, reducing potential side effects. By dynamically adapting the treatment plan to the patient’s changing anatomy, adaptive SABR improves tumor control and patient outcomes, particularly for tumors in mobile areas like the lung or abdomen.
In a 2024 study published in Clinical Oncology, researchers reported on the UK’s first experience with stereotactic magnetic resonance-guided adaptive radiation therapy (SMART) for locally advanced pancreatic cancer (LAPC). Treating 55 patients with 40 Gy in 5 fractions, they observed acceptable toxicity rates, with 71% experiencing only grade 0-1 acute toxicity and no grade >3 acute toxicity. Median overall survival post-diagnosis was 19 months, and one-year local control post-SMART was 65%, demonstrating SMART’s potential to safely deliver ablative doses.
What Are the Main Applications of SABR?
SABR has emerged as a versatile treatment modality with significant applications across a spectrum of cancers and conditions. Notably, it has proven highly effective in addressing early-stage non-small cell lung cancer, particularly for patients who are not suitable candidates for surgery. In prostate cancer, SABR offers a curative option for localized tumors, demonstrating comparable outcomes to traditional radiation therapy and surgery. Furthermore, SABR is increasingly utilized in the management of metastatic cancers, especially those that have spread to a limited number of sites, such as the lung, liver, bone, and spine. In these instances, SABR aims to control tumor growth, alleviate symptoms, and potentially prolong survival.
SABR for Early-Stage Lung Cancer
SABR cures early-stage lung cancer in non-surgical patients. High-dose, precise radiation targets the tumor, minimizing healthy tissue damage. Advanced imaging tracks tumor movement for accuracy. Few treatments offer comparable cure rates to surgery, with less risk and faster recovery.
Wolf et al. (2024), published in Seminars in Thoracic and Cardiovascular Surgery, reviewed SABR (SBRT/SRS) for high-risk stage I NSCLC patients. Analyzing 16 prospective and 14 retrospective studies involving 54,697 patients, they found SABR primarily used for medically inoperable cases (93-95%). Common dosing regimens were 48-66 Gy in 3-5 fractions, with a median 30-month follow-up. The review summarized complications, oncological results, and quality of life after SABR, highlighting the need for ongoing randomized trials comparing SABR to sublobar resection.
SABR for Prostate Cancer
For localized prostate cancer, SABR offers a shorter, more precise treatment. It delivers high-dose radiation directly to the tumor in fewer sessions than traditional methods, enhancing patient convenience. Advanced imaging minimizes damage to surrounding tissues, reducing side effects. SABR achieves similar cure rates to conventional treatments, but avoids surgical risks and allows for faster recovery. This makes it a well-tolerated, efficient option.
Hannan et al. (2022), published in the International Journal of Radiation Oncology Biology Physics, conducted a phase I trial escalating SABR doses for high-risk prostate cancer. They safely escalated to 47.5 Gy (prostate), 55 Gy (MRI lesions), and 25 Gy (pelvic nodes) in 5 fractions, without dose-limiting toxicities. Acute and late grade 2 GU toxicities were 25% and 20%, respectively.
SABR for Metastatic Cancers
SABR plays a crucial role in managing oligometastatic cancers, where cancer has spread to a limited number of sites. By delivering high doses of radiation precisely to these metastatic tumors, SABR can effectively control their growth and potentially eradicate them. For patients with limited metastases in organs like the lung, liver, bone, or spine, SABR can offer significant benefits. In some cases, it can prolong survival by preventing further spread of the disease. Furthermore, by alleviating symptoms such as pain or discomfort caused by metastatic tumors, SABR enhances patient comfort and overall well-being. This focused treatment strategy allows for aggressive management of limited metastatic disease, potentially transforming it into a more manageable, chronic condition.
Doyle et al. (2024), published in Radiotherapy and Oncology, reviewed SABR for oligoprogressive disease. Analyzing 33 studies, they found median progression-free survival (PFS) >6 months in oligoprogressive prostate, non-small cell lung cancer, and renal cancer patients. They concluded SABR shows clinical benefit, but optimal management requires further prospective randomized trials.

Read OncoDaily’s Special Article About Stereotactic Radiotherapy( SBRT)
What to Expect During SABR?
Before SABR, detailed CT and MRI scans map the tumor, allowing for a personalized treatment plan. Patient positioning is vital; immobilization devices ensure stillness during treatment. During sessions, lasting 30-60 minutes, the patient lies still as the linear accelerator delivers high-dose radiation. Real-time imaging may verify accuracy. The radiation therapy team monitors the process. Treatment typically involves 1-5 sessions, far shorter than conventional radiation. Minimal side effects are expected post-treatment due to SABR’s precision.
SABR Side Effects: What Should You Expect?
While SABR minimizes side effects compared to conventional radiation, patients may experience some short-term and long-term effects. These depend on tumor location, radiation dose, and individual factors. Due to SABR’s focused nature, most experience fewer and milder side effects. Short-term effects, if present, are usually mild, like fatigue or skin irritation. Long-term side effects are rare, varying by treated area. Severe side effects are infrequent, and healthcare teams monitor patients to manage issues, ensuring SABR’s benefits outweigh risks.
Olson et al. (2022), published in JAMA Oncology, conducted a phase 2 trial assessing SABR toxicity in 381 patients with oligometastatic/oligoprogressive disease. They reported grade 2 toxic effects in 14.2%, grade 3 in 4.2%, and grade 5 in 0.3% of patients. The cumulative incidence of grade 3 or higher toxic effects at 2 years was 4%. They concluded SABR for oligometastases has acceptable toxicity rates.
Short-Term Side Effects of SABR
Short-term side effects of SABR are typically mild and transient. Common effects include fatigue and localized skin reactions. Fatigue can be managed by ensuring adequate rest and maintaining a balanced diet, rich in nutrients. Localized skin reactions, such as redness or irritation, can be alleviated with gentle skin care, avoiding harsh soaps or lotions. Applying cool compresses and wearing loose-fitting clothing can also provide comfort. It’s important to stay hydrated and follow the healthcare team’s recommendations for managing any discomfort. While most short-term effects resolve quickly after treatment, reporting any persistent or severe symptoms to the medical team is essential.
Long-Term Side Effects of SABR
Long-term side effects from SABR, though less common than short-term effects, can develop months or years after treatment. These late effects might include fibrosis, which is the scarring of tissue, or changes in organ function, depending on the treated area. To minimize these risks, regular follow-up appointments with the healthcare team are essential. These appointments allow for early detection and management of any potential long-term effects This includes a balanced diet, regular exercise, and avoiding smoking, all of which can support overall health and reduce the risk of complications. Regular monitoring through imaging and blood tests, as recommended by the medical team, helps track organ function and identify any changes.
D. Keilty’s study on SAbR for metastatic renal cell carcinoma (mRCC) revealed that long-term toxicities, while generally manageable, included late grade 1-2 effects like fractures, pain, and pneumonitis, with fractures being the most common. Late grade 3-4 toxicities were less frequent, with hyperglycemia occurring more than once. These findings underscore the importance of long-term monitoring for potential complications following SAbR, despite its overall effectiveness in achieving durable local control.
source: www. pancreaticcancer.org.uk
Pros and Cons of SABR
SABR’s advantages include non-invasive delivery of high-dose radiation, fewer sessions, and minimal healthy tissue damage. For early-stage lung cancer, it matches surgery outcomes, avoiding invasive risks. In localized prostate cancer, SABR offers similar cures with fewer treatments. However, it’s unsuitable for large or critically located tumors. An elderly patient with a small lung tumor benefits greatly, while a large, invasive tumor may require surgery or combined therapies. Testimonials emphasize SABR’s convenience and minimal disruption.
Who Is a Good Candidate for SABR?
Ideal SABR candidates have small, defined tumors, often early-stage or limited metastatic. Compared to surgery, SABR is non-invasive, avoiding anesthesia and surgical risks, especially for elderly or frail patients. Efficacy is comparable to surgery in some cancers, like early lung and localized prostate. Recovery is quicker than surgery, allowing faster return to normal activities. Compared to conventional radiotherapy, SABR delivers higher doses in fewer sessions. Side effects are generally fewer and milder due to precise targeting. Patient preference is key; many choose SABR for convenience and reduced complications. For example, a patient with a small lung tumor, unsuitable for surgery due to comorbidities, might be a good SABR candidate.
Innovations in SABR
Following stereotactic ablative radiotherapy (SABR), the recovery process is typically straightforward, with most patients experiencing minimal disruption to their daily lives. Due to the precise nature of SABR, activity restrictions are generally limited, and patients can often resume their normal routines shortly after treatment. Managing potential side effects, which are usually mild, involves addressing any localized discomfort or fatigue that may arise. Healthcare teams provide guidance on managing these effects, which might include recommendations for rest, hydration, or mild pain relief.
Cilla et al. (2024), published in Radiotherapy and Oncology, developed and validated machine learning (ML) models to predict complete response (CR) after SBRT for oligometastatic gynecological cancer. Using data from 157 patients (272 lesions), they found lesion volume (PTV), lesion type, and biological effective dose (BED10) were key predictors. SVM with a radial basis function kernel achieved an AUC of 0.800 in the training set and 0.771 in external validation, showing reliable prediction of treatment response.
Recovery of the Body After SABR
Post-SABR recovery generally involves minimal disruption to daily life, as activity restrictions are typically few. Managing potential side effects, such as mild discomfort or fatigue, is usually straightforward, with healthcare teams providing guidance on rest, hydration, or mild pain relief. It’s crucial for patients to recognize signs that necessitate medical attention, including severe pain, persistent nausea, unexpected bleeding, or significant changes in bodily functions. While SABR is designed to minimize damage to healthy tissues, any unusual symptoms should be promptly reported to the healthcare team for appropriate management. Regular follow-up appointments are scheduled to monitor recovery and treatment effectiveness, allowing for timely intervention if needed.
Written by Aren Karapetyan, MD
FAQ
What is SABR?
SABR (Stereotactic Ablative Radiotherapy) is a precise radiation therapy that delivers high doses of radiation to tumors, minimizing damage to healthy tissue.
How does SABR differ from conventional radiation therapy?
SABR uses higher doses in fewer sessions, with more precise targeting, compared to conventional radiation.
What types of cancer can SABR treat?
SABR can treat early-stage lung, prostate, kidney, liver, bone, and metastatic cancers.
How many treatments are typically involved in SABR?
Usually, 1 to 5 treatments.
Is SABR painful?
No, SABR is a painless procedure.
What are the common side effects of SABR?
Side effects are typically minimal, such as fatigue or localized skin irritation, and less than conventional radiation
Is SABR a surgical procedure?
No, SABR is a non-invasive radiation therapy.
How accurate is SABR?
SABR is very accurate, using advanced imaging and immobilization to achieve millimeter-scale precision.
Who is a good candidate for SABR?
Patients with small to moderately sized, well-defined tumors, especially those unsuitable for surgery.
What is the recovery time after SABR?
Recovery is typically quick, with minimal disruption to daily life.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
