Fresh from the stage at ESTRO 2025, the Danish Breast Cancer Group (DBCG) SKAGEN Trial 1 investigated the use of moderately hypofractionated adjuvant loco-regional radiation therapy (RT) in patients with early high-risk unilateral breast cancer. Conducted between 2015 and July 2021, the trial compared a standard RT regimen of 50 Gy in 25 fractions with an experimental regimen of 40 Gy in 15 fractions. Eligibility criteria were broad, including any patient indicated for loco-regional RT, with no prior cancer history and a willingness to commit to a 10-year follow-up.
Study design and methods of SKAGEN trial 1
The trial enrolled 2,963 patients from 17 institutions in 7 countries. Of these, 8 institutions, contributing 2,184 enrolled patients, also provided data on the total number of patients treated with loco-regional RT during the trial period (n = 6,929).
Across these institutions, the overall TPR was 31.5%, with annual, site-specific TPRs ranging from 14.4% to 50.4%. All participating institutions began accrual in 2016, and trends revealed a modest decline in participation during 2019–2020, coinciding with the COVID-19 pandemic. In one institution, patient enrollment was halted in 2020 due to pandemic-related restrictions. However, by 2021, TPRs began to stabilize.

Results
With a median follow-up of 4.1 years, the DBCG Skagen Trial 1 provided a detailed analysis of both safety and efficacy outcomes comparing 40 Gy in 15 fractions to the standard 50 Gy in 25 fractions for loco-regional radiotherapy in breast cancer. The primary endpoint – arm lymphedema – was assessed dynamically across multiple time points, along with secondary endpoints including recurrence patterns and mortality.
Lymphedema (Primary Endpoint)
- Prevalence is dynamic over time—some patients improve or worsen between follow-ups.
- No significant difference in lymphedema risk between 40 Gy and 50 Gy arms.
- Axillary surgery (not fractionation) was the main driver:
- Hazard ratio for lymphedema with axillary lymph node dissection: 3.16
- No interaction between fractionation and type of surgery (sentinel vs. ALND)
Patterns of Failure
- Loco-regional recurrence: No significant difference between groups- HR: 0.86 (crosses 1.0)
- Distant recurrence: No difference HR: 1.10 (crosses 1.0)
Mortality Outcomes
- All-cause mortality: Slightly higher in the 40 Gy arm
- HR: 1.27 (borderline significance, lower CI = 0.98)
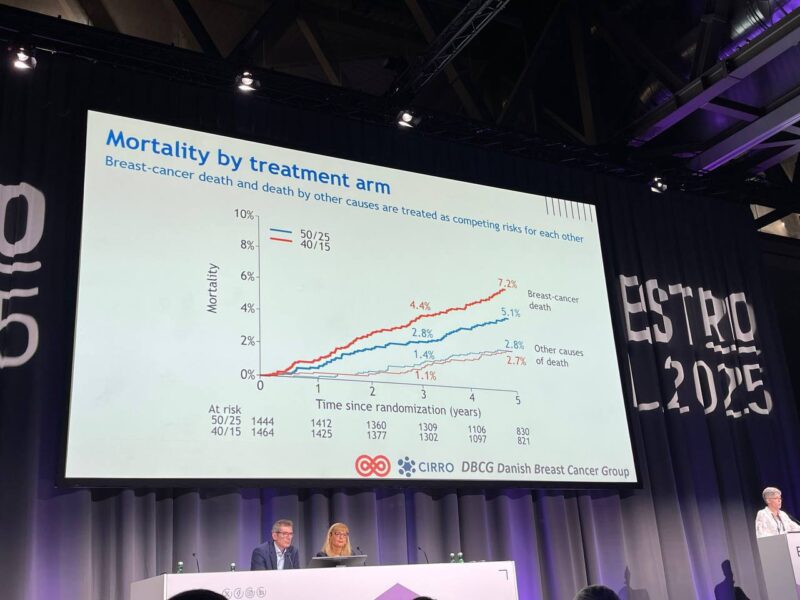
Breast cancer–specific mortality:
- HR: 1.44, suggesting a potential increased risk in the 40 Gy group
- Derived from competing risk analysis, accounting for non-breast cancer deaths
Key takeaways
The findings underscore substantial variability in trial enrollment across institutions and highlight that, despite the logistical appeal of a shorter RT course, no more than one center enrolled over half of its eligible patients. This suggests that logistical feasibility alone is insufficient to guarantee high participation. The authors advocate for systematic TPR monitoring and data collection on barriers to enrollment to inform the design and execution of future radiotherapy trials.
• At 4.1 years follow up, 40Gy/15 does not cause more arm lymphedema compared with 50Gy/25
• The risk of loco-regional failure, distant failure and death from any cause not higher with 40Gy/15 compared with 50Gy/25. BCM higher using 40Gy/15, concerning
• BCM worse with 40Gy/15 in all sub-groups tested
• 40Gy/15 became DBCG standard for loco-regional RT 2021
• Publication is planned in close collaboration with the group of Sofia Rivera, HYPO G-01 trial (1200 patients treated in a similar trial)
• Close monitoring of the follow up in the trial continues in order to determine the long term safety of moderately hypofractionated loco-regional RT of high-risk breast cancer patient
Dr. Meattini Icro, who summarized both the Skagen and FAST-Forward trials at ESTRO 2025, emphasized that while hypofractionated regional RT is already recommended in national guidelines (e.g., Spain 2021, ARO 2022), broader international uptake and guideline expansion is expected following trials like SKAGEN. Trial participation needs to be improved, especially in high-risk patients and complex treatment settings.
Read ESTRO 2025 Updates on OncoDaily.



