Platinum-resistant and platinum-refractory ovarian cancer remains one of the most challenging settings in gynecologic oncology. Historical trials of immune checkpoint inhibitors, including CTLA-4 and PD-1 blockade, have shown limited activity, with objective response rates typically below 10% and no regulatory approvals to date. The immunologically “cold” tumor microenvironment of ovarian cancer has been a major barrier to effective immunotherapy.
Agenus has developed Botensilimab, an Fc-enhanced anti–CTLA-4 antibody designed to improve engagement of activating Fcγ receptors, augment antigen presentation, and deplete regulatory T cells. When combined with Balstilimab, a fully human anti–PD-1 antibody, this strategy aims to overcome immune resistance through complementary mechanisms of immune activation.
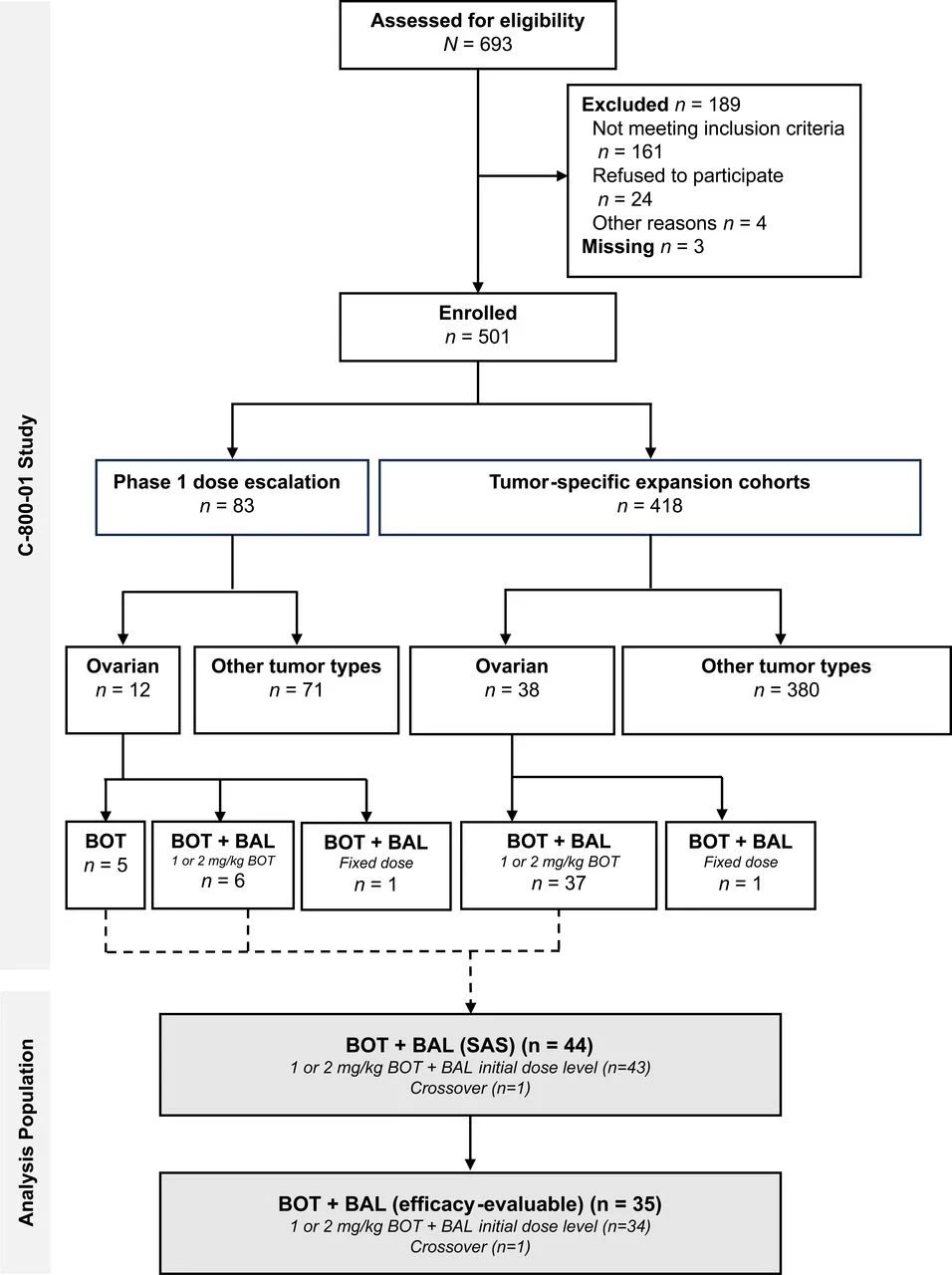
Study Design and Methods
The C-800–01 trial is an open-label, multicenter phase 1b study evaluating botensilimab with or without balstilimab in treatment-refractory solid tumors. The ovarian cancer expansion cohort enrolled heavily pretreated patients, most of whom had platinum-resistant or platinum-refractory disease.
Patients received botensilimab at 1 or 2 mg/kg every 6 weeks combined with balstilimab 3 mg/kg every 2 weeks. Primary objectives focused on safety and tolerability, while secondary and exploratory endpoints included objective response rate, duration of response, progression-free survival, overall survival, and immune-correlative biomarkers. Tumor responses were assessed per RECIST v1.1.
Results
Among 44 treated patients, 35 were evaluable for efficacy. The population was highly pretreated, with a median of three prior lines of therapy, universal prior platinum exposure, and frequent prior use of bevacizumab and PARP inhibitors.
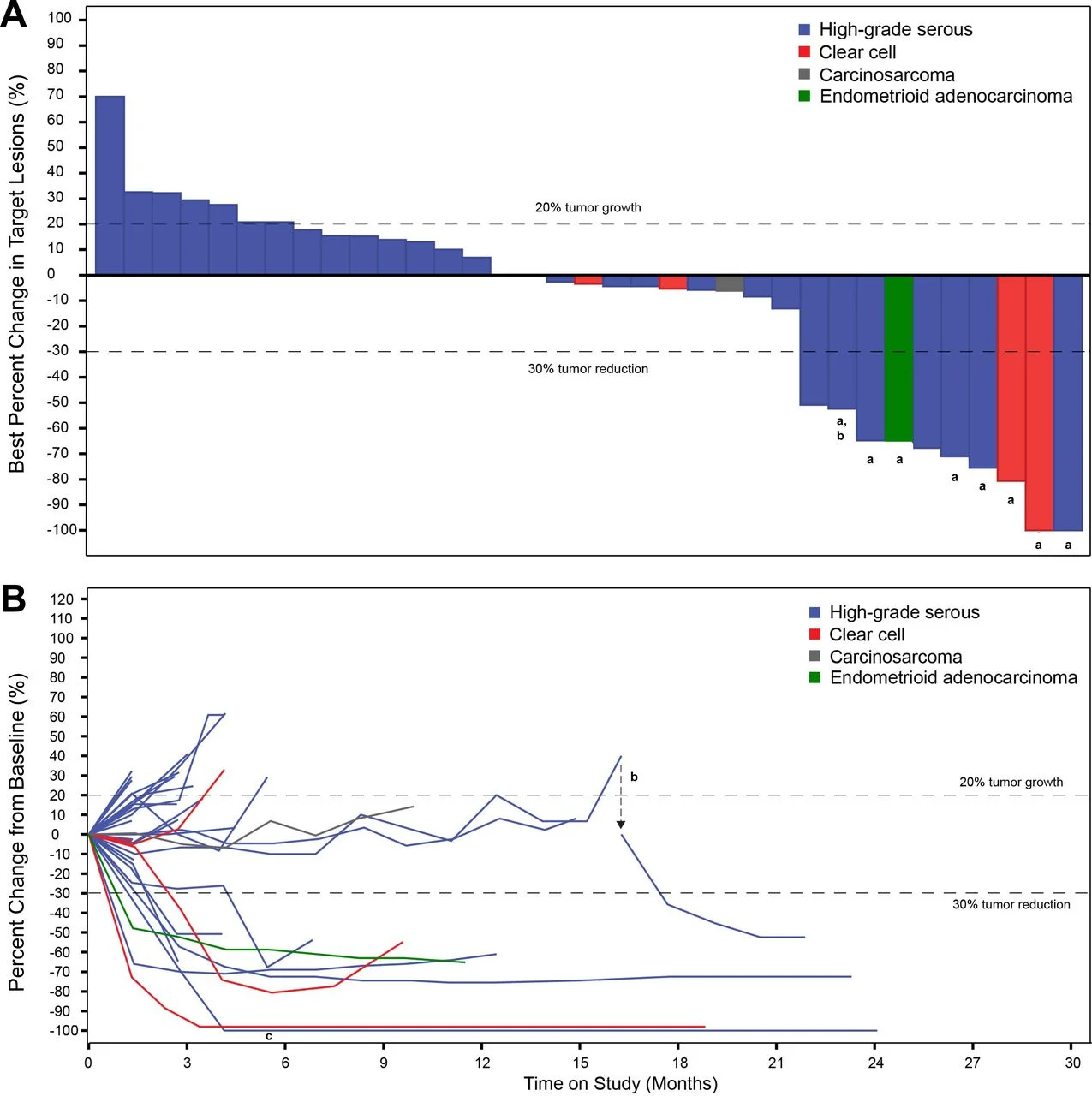
The confirmed objective response rate was 23%, including one complete response and seven partial responses. Importantly, responses were deep and durable, with a median duration of response of 9.7 months, and several patients maintaining benefit well beyond radiographic progression. The clinical benefit rate—defined as complete response, partial response, or stable disease lasting at least 24 weeks—reached 31%, highlighting that RECIST criteria underestimated meaningful benefit in this cohort.
Median progression-free survival was 2.8 months, while median overall survival reached 14.8 months, with a 12-month overall survival rate of 75%, a notable outcome in this heavily pretreated population.
Safety
Treatment-related adverse events were common but largely manageable. Immune-mediated diarrhea and colitis were the most frequent toxicities, occurring in 43% of patients, with grade 3 events in 16%. No treatment-related deaths were reported.
A key aspect of this study was the proactive use of tumor necrosis factor-alpha inhibitors, such as infliximab, for steroid-responsive immune-mediated colitis. This approach enabled faster resolution of toxicity, minimized prolonged steroid exposure, and allowed selected patients to safely resume therapy—reflecting an evolving paradigm in immune-related adverse event management.
Biomarker Insights
Comprehensive immune profiling revealed that clinical benefit was strongly associated with a T-cell–inflamed tumor microenvironment. Responders and patients with durable stable disease more frequently exhibited T-cell infiltrated phenotypes, higher densities of CD8+ cytotoxic T cells, and the presence of tertiary lymphoid structures.
Notably, responding tumors showed higher levels of FcγRIIIA+CD11c+ immune cells, consistent with the Fc-enhanced mechanism of botensilimab. PD-L1 expression was higher in responders and correlated with immune infiltration, although it was not independently predictive of survival. Tumor mutational burden and baseline inflammatory markers, including IL-6, showed limited prognostic value.
Key Takeaway Messages
- Botensilimab plus balstilimab produced clinically meaningful and durable responses in treatment-refractory ovarian cancer.
- Benefit extended beyond RECIST responses, with prolonged stable disease in a substantial subset of patients.
- Toxicities were manageable and reversible, supported by modern immune-toxicity management strategies.
- Immune-correlative analyses support the biological rationale of Fc-enhanced CTLA-4 blockade and highlight potential biomarkers for future patient selection.
You can read all article here