Novartis has released new phase 3 results showing that its investigational antibody ianalumab, when added to Promacta (eltrombopag), can significantly prolong disease control in adults with primary immune thrombocytopenia (ITP) who have previously been treated with corticosteroids.
In the VAYHIT2 trial, patients received either ianalumab or placebo on top of eltrombopag, a standard second-line therapy in ITP. The study evaluated two intravenous doses of ianalumab (9 mg/kg and 3 mg/kg), given as four once-monthly infusions, compared with placebo, all in combination with daily eltrombopag.
Extended disease control with four monthly doses
The primary endpoint was time to treatment failure, a composite measure capturing how long patients maintained safe platelet counts without needing rescue therapy, additional ITP treatment, or experiencing significant bleeding events.
Ianalumab 9 mg/kg plus eltrombopag extended ITP disease control by about 45% compared to placebo plus eltrombopag. Patients in the higher-dose ianalumab arm maintained disease control a median of 13.0 months versus 4.7 months in the placebo arm, meaning they stayed controlled roughly 2.8 times longer.
Both ianalumab doses met the primary endpoint, with hazard ratios around 0.55–0.58 versus placebo, indicating a meaningful reduction in the risk of treatment failure over time.
Higher sustained platelet responses and improved fatigue
The trial also met a key secondary endpoint. At six months, 62% of patients treated with ianalumab 9 mg/kg plus eltrombopag achieved a sustained platelet response, compared with 39% of those receiving placebo plus eltrombopag. The 3 mg/kg dose also showed a numerically higher sustained response rate, supporting efficacy across both dosing strategies.
Patient-reported outcomes suggested an added benefit beyond platelet counts. Fatigue scores, measured using the PROMIS Fatigue instrument, improved more in the ianalumab arm than in the placebo arm, with a greater mean reduction in fatigue severity over time.
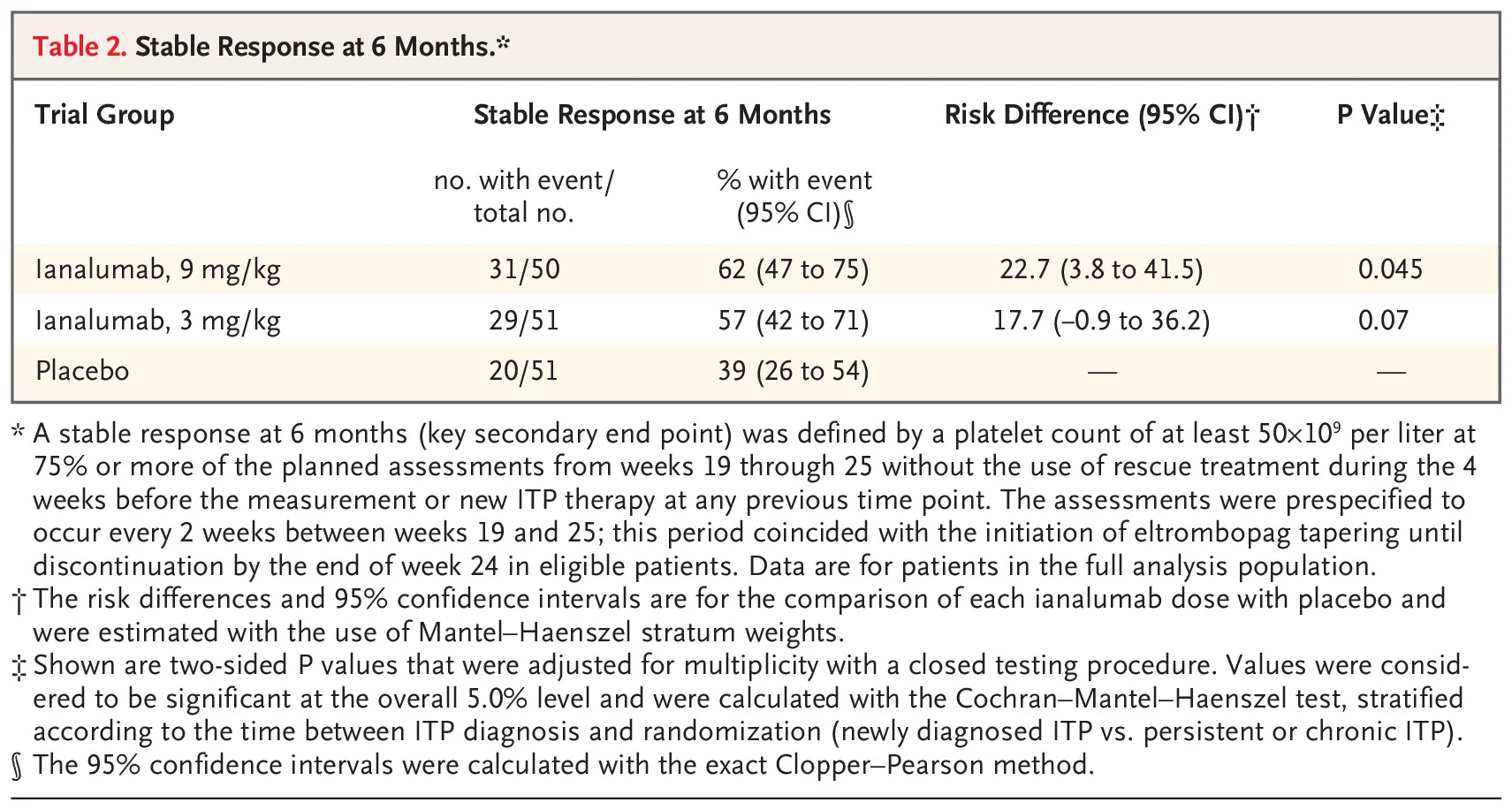
Stable Response at 6 Months
source: www.nejm.org
A potential disease-modifying approach in ITP
Ianalumab targets the BAFF receptor on B cells, aiming both to deplete B cells and block key survival signals. This dual mechanism is designed to address the autoimmune process that leads to platelet destruction in ITP, potentially moving beyond purely symptomatic, count-raising approaches.
In VAYHIT2, patients received only four monthly infusions of ianalumab, after which eltrombopag could be tapered and discontinued if platelet responses were adequate. Many patients were then followed off active treatment, offering a glimpse of more durable disease control without continuous therapy.
Read Our Special Article About Differences Of Immunotherapy and Targeted Therapy
Next steps and regulatory path
According to Novartis, detailed VAYHIT2 data are being presented in a Late-Breaking Abstract session at the 67th American Society of Hematology (ASH) Annual Meeting and published simultaneously in The New England Journal of Medicine.
The company plans to submit the VAYHIT2 results in second-line ITP, together with findings from the ongoing first-line ITP trial VAYHIT1, to health authorities in 2027. If approved, ianalumab could offer a time-limited, B-cell-directed option that extends disease control and reduces the need for chronic treatment in people living with ITP.


