Adjuvant immunotherapy has significantly reduced recurrence risk in resected melanoma, yet recent phase III trials combining immune checkpoint inhibitors in the postoperative setting have failed to improve outcomes beyond PD-1 monotherapy. In contrast, neoadjuvant immunotherapy consistently demonstrates superior efficacy across melanoma and other solid tumors. This discrepancy suggests fundamental biological differences between neoadjuvant and adjuvant contexts. Following surgical resection, minimal residual disease is characterized by low antigen burden and limited tumor–immune interaction, potentially constraining effective T-cell priming. Emerging evidence indicates that successful adjuvant strategies may require active enhancement of tumor-antigen priming—rather than additional checkpoint blockade alone—to generate durable antitumor immunity.
Why Neoadjuvant Beats Adjuvant
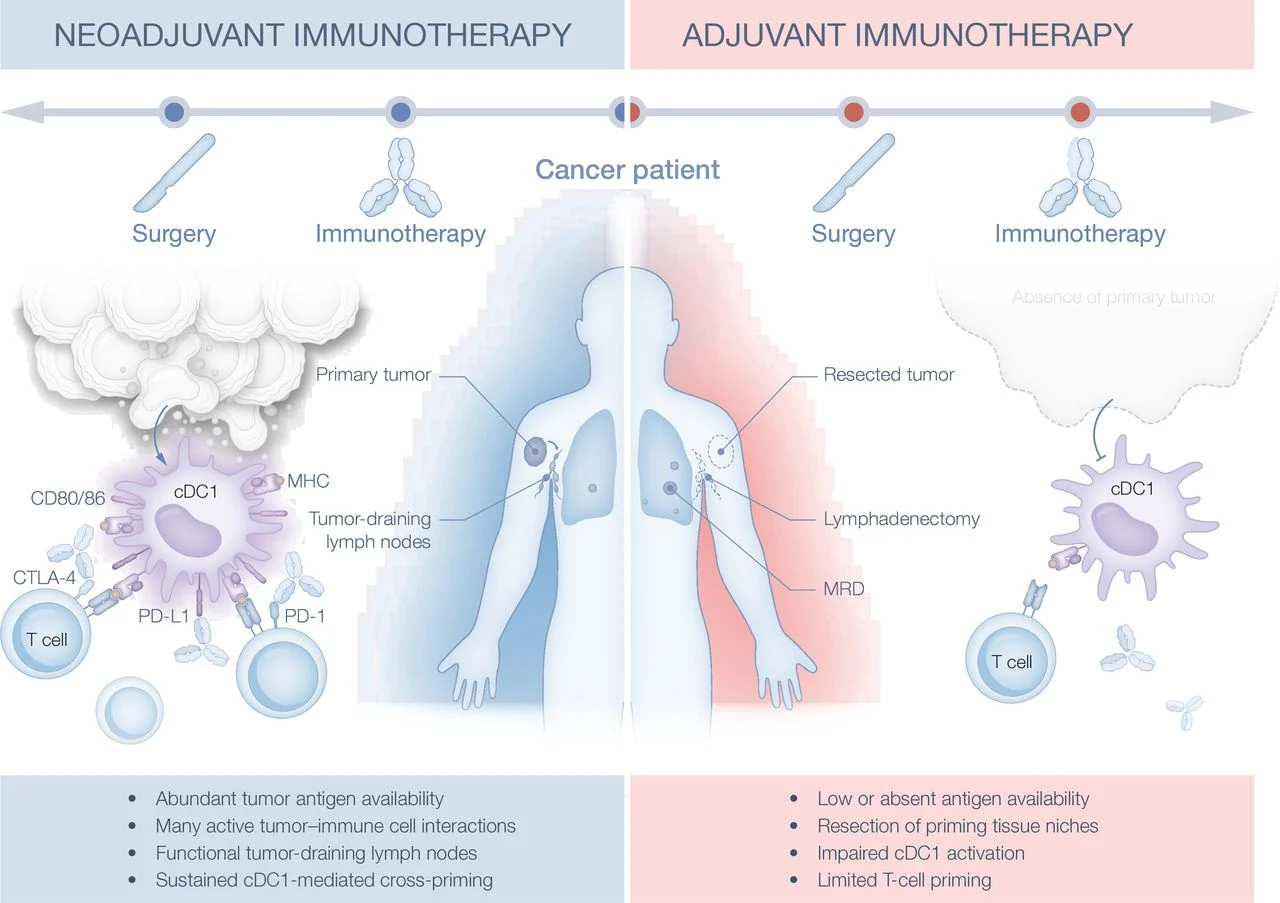
Checkpoint inhibitors consistently perform better when given before surgery, across melanoma and other solid tumors. The key difference is antigen availability: with the tumor in place, there is continuous tumor-antigen cross-presentation—primarily by cDC1 dendritic cells—that fuels robust CD8⁺ T-cell priming. In contrast, the adjuvant (MRD) setting is antigen-poor; PD-1/PD-L1 blockade has little to amplify when signal 1 (antigen) is weak or absent.
Why Dual Checkpoint Blockade Failed in MRD
Large phase III adjuvant trials combining PD-1 with CTLA-4 or LAG-3 (eg, CheckMate 915, RELATIVITY-098, KeyVibe-010) did not improve relapse-free survival over PD-1 monotherapy. The implication is biological—not pharmacologic: adding another checkpoint cannot compensate for insufficient priming in MRD. Checkpoint inhibitors potentiate signal 2 (costimulation) but do little without adequate signal 1.
cDC1 as Central Orchestrators of Antitumor T-Cell Priming
A growing body of preclinical and translational data identifies conventional type 1 dendritic cells (cDC1) as critical determinants of effective antitumor immunity. cDC1 specialize in cross-presentation of tumor-derived antigens and provide essential cytokine and costimulatory signals, including IL-12, CD40/CD40L, CD137 (4-1BB), and IL-15, which are required for optimal activation, expansion, and maintenance of cytotoxic T lymphocytes. Importantly, cDC1 sustain intratumoral pools of TCF1⁺ stem-like CD8⁺ T cells, a population now recognized as indispensable for durable responses to PD-1 blockade.
Functional studies in murine tumor models demonstrate that selective depletion of cDC1 abrogates the therapeutic activity of immune checkpoint inhibitors, establishing a causal role for these cells in mediating treatment efficacy. Consistently, higher intratumoral cDC1 density and transcriptional signatures associated with cDC1 activity correlate with improved clinical outcomes across multiple cancer types, including melanoma. In the adjuvant setting, surgical lymphadenectomy may further compromise immune priming by eliminating tumor-draining lymph nodes that serve as critical cDC1-dependent priming niches, thereby limiting the effectiveness of checkpoint blockade in minimal residual disease.
What Does Work in the Adjuvant Setting—and Why
Adjuvant strategies that succeed share a common feature: they prime.
- Anti-PD-1 monotherapy improves RFS (eg, nivolumab, pembrolizumab), but benefits plateau in MRD.
- BRAF/MEK inhibitors (in BRAF-mutant melanoma) likely help via immunogenic modulation—increasing antigen presentation and T-cell infiltration (COMBI-AD).
- Personalized mRNA vaccines (eg, KEYNOTE-942) provide exogenous antigen, expand neoantigen-specific CD8⁺ T cells, and show encouraging adjuvant signals—precisely because they recreate priming after resection.
Implications for Trial Design
Future adjuvant programs should be mechanism-first, not empirical:
- Prime first, modulate second. Pair PD-1 blockade with agents that create antigenic stimulus or expand/activate cDC1.
- Prioritize biomarkers (cDC1 infiltration, IFN-γ signatures, TCR dynamics).
- Consider sequencing and anatomy (timing of lymph node surgery; preserving priming sites).
- Promising priming strategies: FLT3L to expand cDC1, intratumoral TLR/STING agonists, and targeted mRNA platforms delivering antigen to XCR1⁺/CLEC9A⁺ dendritic cells.
IO Takeaway
Adjuvant immunotherapy must evolve from passive maintenance to active immunization. The failure of dual checkpoint blockade in MRD is not a lack of synergy—it is a lack of upstream immune activation. Priming is the bottleneck. Get priming right, and checkpoint inhibition can deliver.
You Can Watch More on OncoDaily Youtube TV


