Hormone receptor–positive, HER2-negative (HR+/HER2–) breast cancer represents the largest subtype of early breast cancer but remains relatively immunologically “cold,” characterized by low PD-L1 expression, sparse tumor-infiltrating lymphocytes (TILs), and modest tumor mutational burden. Despite this, multiple randomized trials—including I-SPY2, KEYNOTE-756, and CheckMate 7FL—have demonstrated that a subset of patients derives meaningful benefit from adding immune checkpoint inhibition to neoadjuvant chemotherapy. However, optimal patient selection, biomarker enrichment, and treatment sequencing remain unresolved.
Study Design
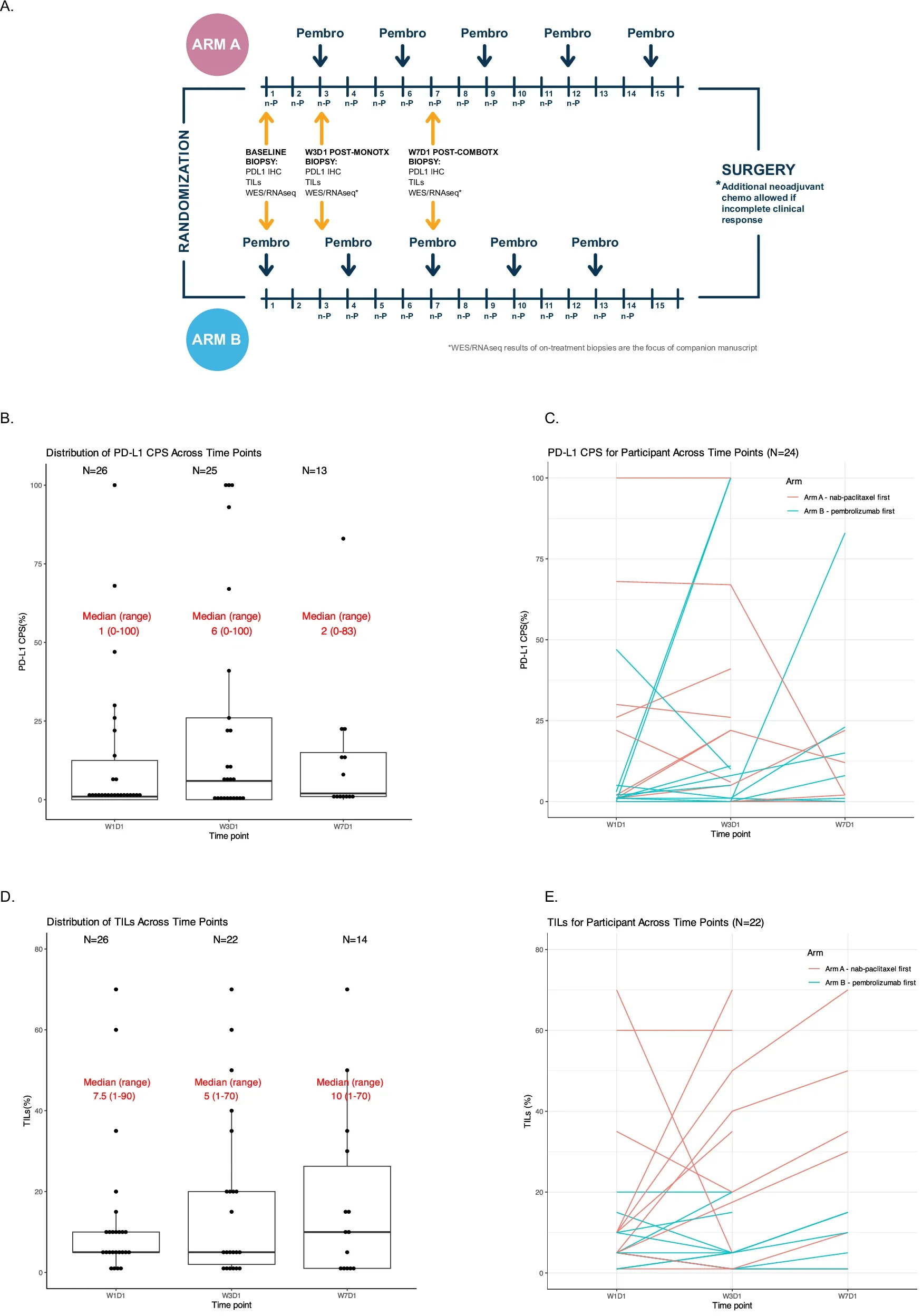
This randomized pilot trial (NCT02999477) evaluated neoadjuvant nab-paclitaxel plus pembrolizumab in stage II–III HR+/HER2– breast cancer, incorporating a unique run-in design to explore biological effects of treatment sequencing. Patients were randomized 1:1 to receive a 2-week window of either nab-paclitaxel or pembrolizumab monotherapy, followed by combination therapy. Serial tumor biopsies enabled dynamic immune and genomic profiling. The primary endpoint was change in PD-L1 expression after run-in therapy.
Key Efficacy Findings
- Pathologic complete response (pCR): 17% overall
- Residual cancer burden (RCB) 0–1: 28% (including patients who received additional anthracycline)
- Radiographic response rate: 80%
- 3-year event-free survival: 86% (95% CI, 69–100%)
While pCR rates were lower than those reported in larger phase III trials, this cohort included a broader biological spectrum, with fewer grade 3 tumors, inclusion of lobular histology, and incomplete anthracycline exposure—factors known to influence response.
Primary Endpoint: PD-L1 Modulation
The trial did not meet its primary endpoint. Neither chemotherapy nor pembrolizumab run-in significantly increased PD-L1 expression, whether assessed by tumor cell H-score or Combined Positive Score (CPS). This finding challenges the assumption that short-term immune or chemotherapy priming reliably inflames HR+ tumors.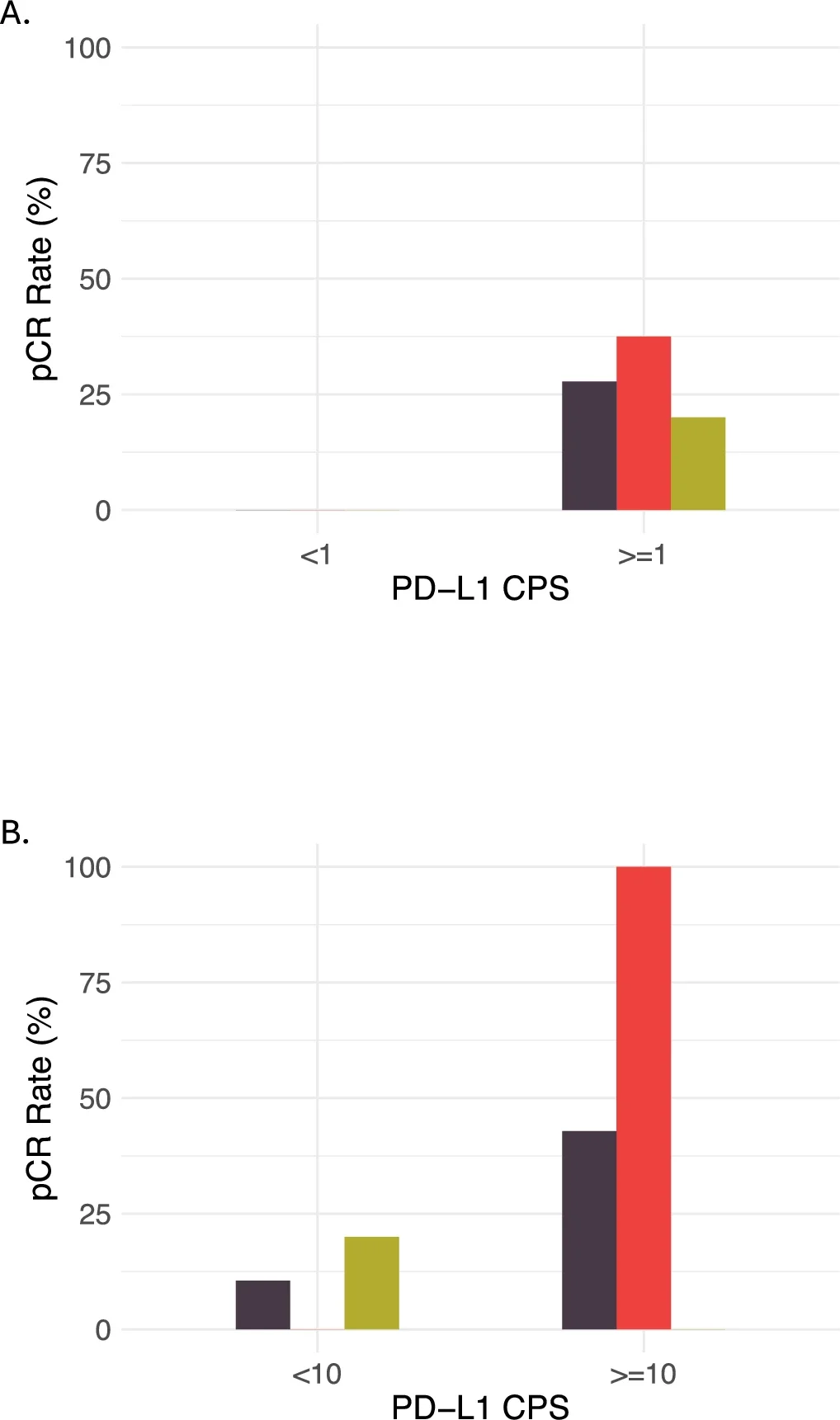
Translational Biomarker Insights
Despite limited PD-L1 dynamics, baseline immune biology strongly predicted response:
Favorable response (RCB 0–1) was associated with:
- Higher baseline PD-L1 CPS
- Enrichment of inflammatory gene signatures (interferon-γ, interferon-α, TNF-α/NF-κB, allograft rejection)
- Increased CD8⁺ T cells, activated memory CD4⁺ T cells, and M1-like macrophages
- Pro-inflammatory cytokine activity (IL-1α/β, TNF-α, IL-27)
Unfavorable response (RCB II–III) was associated with:
- Strong estrogen response gene expression (early and late)
- Reduced antigen processing and presentation pathways
- A negative correlation between estrogen signaling and immune visibility
These findings reinforce a central theme in HR+ breast cancer: estrogen-dominant biology may actively suppress immune recognition, limiting the effectiveness of immunotherapy-containing regimens.
Sequencing Matters—Biology Differs by Run-In
Although clinical outcomes did not differ by treatment order, distinct biological programs emerged:
- Chemotherapy-first responders showed enrichment of antigen presentation, interferon signaling, and checkpoint-related genes.
- Immunotherapy-first responders exhibited immune cell–intrinsic activation signatures (e.g., CD8A, CD40, BANK1).
This suggests that mechanisms of response differ depending on whether immune activation or tumor cytotoxicity is initiated first, a hypothesis warranting further study.
Safety Profile
The regimen was generally manageable:
- Grade ≥3 immune-related AEs: 13%
- Common toxicities reflected nab-paclitaxel exposure
- No grade 5 events
- Over half of patients discontinued pembrolizumab early, largely due to toxicity
These data underscore the need for careful patient selection when extending immunotherapy to less immunogenic breast cancer subtypes.
Emerging Role of the Microbiome
Exploratory analyses suggested associations between gut microbial metabolic pathways (notably arginine and nucleotide synthesis) and treatment response. While hypothesis-generating, these findings align with growing evidence that host–microbiome–immune interactions influence immunotherapy outcomes.
Key Takeaway Messages
- Neoadjuvant pembrolizumab plus chemotherapy is biologically active in a subset of HR+/HER2– breast cancers.
- Baseline immune inflammation—not on-treatment PD-L1 induction—drives benefit.
- Estrogen signaling emerges as a dominant resistance pathway, linked to impaired antigen presentation.
- Treatment sequencing alters immune biology, even when clinical outcomes appear similar.
- Precision immunotherapy in HR+ breast cancer will require biomarker-driven selection, not broad application.

10 ongoing Clinical Trials on Immunotherapy in Breast Cancer
You Can Read All Article Here


