At ASCO GI 2026, Peter R. Galle presented the updated 4-year follow-up results of the phase III CheckMate 9DW trial, a pivotal study comparing nivolumab plus ipilimumab (NIVO + IPI) with lenvatinib or sorafenib (LEN/SOR) as first-line treatment for patients with unresectable hepatocellular carcinoma (HCC). These data provide the longest follow-up to date for dual immune checkpoint blockade in the frontline HCC setting and further clarify its long-term efficacy and safety profile.
Title: Nivolumab plus ipilimumab vs lenvatinib or sorafenib as first-line treatment for unresectable hepatocellular carcinoma (HCC): 4-year follow-up of CheckMate 9DW.
Authors: Peter R. Galle, Bruno Sangro, Thomas Decaens, Masatoshi Kudo, Shukui Qin, Leonardo Da Fonseca, Hatim Karachiwala, Joong-Won Park, Edward Gane, Matthias Pinter, David Tai, Armando Santoro, Gonzalo Pizarro, Michael Schenker, Qi Wang, Maria Jesus Jimenez Exposito, Thomas Yau.
Study Design and Methods
CheckMate 9DW (NCT04039607) is a global, randomized, open-label, phase III trial enrolling adults with previously untreated, histologically confirmed advanced or unresectable HCC. Patients were required to have preserved liver function (Child–Pugh class A, score ≤6), an ECOG performance status of 0 or 1, and at least one measurable untreated lesion according to RECIST v1.1. Patients had unresectable HCC and were systemic therapy–naïve; they could be ineligible for or have recurrent disease following curative surgery and/or locoregional therapies.
Participants were randomized in a 1:1 ratio to receive either combination immunotherapy or standard tyrosine kinase inhibitor (TKI) therapy. The experimental arm consisted of nivolumab 1 mg/kg plus ipilimumab 3 mg/kg administered every 3 weeks for up to four induction doses, followed by maintenance nivolumab 480 mg every 4 weeks.
Nivolumab treatment was permitted for a maximum duration of two years. The control arm comprised investigator’s choice of lenvatinib (8 mg or 12 mg once daily, weight-based) or sorafenib (400 mg twice daily), administered until disease progression or unacceptable toxicity. Randomization was stratified by disease etiology (HBV vs HCV vs uninfected), presence of macrovascular invasion or extrahepatic spread, and baseline AFP level (<400 vs ≥400 ng/mL).
Endpoints
The primary endpoint was overall survival (OS). Key secondary endpoints included objective response rate (ORR) and duration of response (DOR) assessed by blinded independent central review, time to symptom deterioration, and safety.

Results from ASCO GI 2026
In total, 668 patients were randomized, with 335 patients assigned to the NIVO + IPI arm and 333 patients to the LEN/SOR arm. Among treated patients in the LEN/SOR group, 275 of 325 patients (85%) received lenvatinib. At the time of this analysis, the median follow-up was 52.5 months (range 44.0–66.1), providing a robust assessment of long-term outcomes.
Overall survival continued to favor NIVO + IPI over LEN/SOR. Median OS was 23.7 months (95% CI 18.8–29.4) in the NIVO + IPI arm compared with 20.6 months (95% CI 17.7–22.5) in the LEN/SOR arm, corresponding to a hazard ratio of 0.78 (95% CI 0.65–0.93). Importantly, long-term survival rates highlighted a meaningful tail of the survival curve, with 48-month OS rates of 31% in the NIVO + IPI arm versus 18% in the LEN/SOR arm.
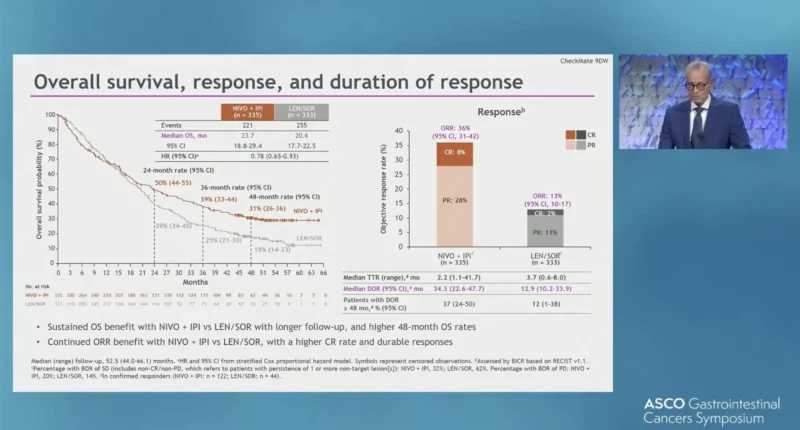
Tumor responses were both more frequent and more durable with combination immunotherapy. The ORR was 36% with NIVO + IPI compared with 13% with LEN/SOR. Complete responses were observed in 8% of patients receiving NIVO + IPI versus 2% in the control arm. Among responding patients, the median DOR was 34.3 months (95% CI 22.6–47.7) with NIVO + IPI, substantially longer than the 12.9 months (95% CI 10.2–33.9) observed with LEN/SOR.
The safety profile remained consistent with prior analyses and no new safety signals emerged with extended follow-up. Any-grade treatment-related adverse events occurred in 83% of patients treated with NIVO + IPI and 91% of those treated with LEN/SOR. Grade 3–4 treatment-related adverse events were reported in 41% and 42% of patients, respectively. Discontinuation due to treatment-related adverse events occurred in 18% of patients in the NIVO + IPI arm (grade 3–4: 13%) and 10% in the LEN/SOR arm (grade 3–4: 6%).
Treatment-related deaths were reported in 4% of patients receiving NIVO + IPI and in <1% of patients in the LEN/SOR arm, consistent with prior analyses. Immune-mediated hepatitis occurred in a subset of patients treated with NIVO + IPI and was generally manageable using established treatment algorithms. Most cases did not lead to permanent treatment discontinuation, and no new safety concerns were observed with longer follow-up.
You can also read about Sorafenib (Nexavar) on OncoDaily.
Conclusion
With more than four years of follow-up, CheckMate 9DW confirms that first-line nivolumab plus ipilimumab delivers durable overall survival and response benefits compared with lenvatinib or sorafenib in unresectable HCC, while maintaining a manageable and well-characterized safety profile. The persistence of long-term survival benefit and deep, durable responses further reinforces NIVO + IPI as a standard-of-care option for appropriately selected patients in the frontline HCC setting.
Read the full abstract here.


