
ATOMIC Trial (Alliance A021502): Evaluating Atezolizumab in Adjuvant Treatment of MSI-H/dMMR Stage III Colon Cancer
Microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR) colorectal cancer represents a distinct biological subset of tumors, known for a high mutation burden and an immunogenic tumor microenvironment. While immunotherapy has revolutionized the treatment of metastatic MSI-H/dMMR colorectal cancer, its role in the adjuvant setting for resected stage III colon cancer has remained undefined until recently. The ATOMIC trial (Alliance A021502) is a landmark phase III study evaluating whether the addition of atezolizumab, an anti–PD-L1 checkpoint inhibitor, to FOLFOX chemotherapy improves outcomes in this molecular subgroup of colon cancer patients.
Read More About Immunotherapy for Colon Cancer on Oncodaily
Background and Rationale
Traditionally, stage III colon cancer is treated with adjuvant chemotherapy—commonly FOLFOX (5-fluorouracil, leucovorin, and oxaliplatin)—to reduce the risk of recurrence. However, patients with dMMR/MSI-H tumors have shown limited benefit from 5-FU monotherapy in stage II disease and uncertain benefit in stage III, raising questions about the optimal approach (Ribic et al., 2003). In metastatic disease, immune checkpoint inhibitors such as pembrolizumab and nivolumab have demonstrated superior efficacy over chemotherapy in MSI-H/dMMR colorectal cancer (Andre et al., 2020).
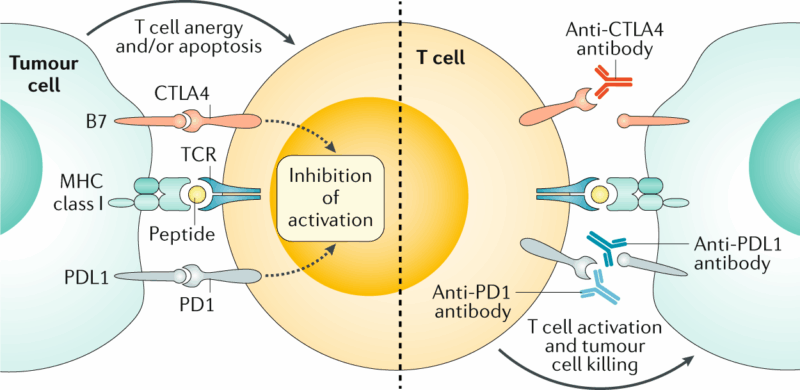
The ATOMIC trial was designed to explore whether this immunological advantage could be translated into improved disease-free survival when immunotherapy is given after surgical resection in stage III disease.
Study Design
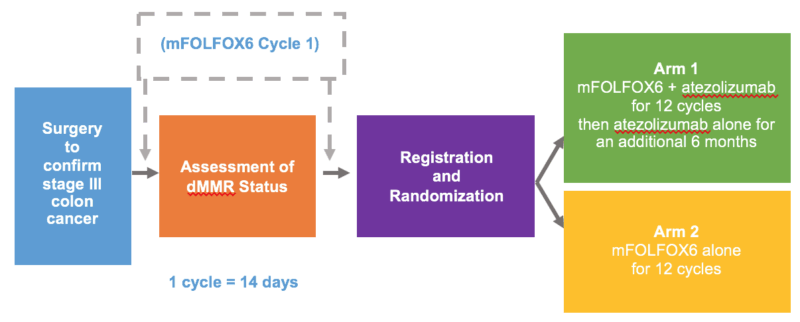
The ATOMIC trial is a randomized, open-label, phase III multicenter study sponsored by the Alliance for Clinical Trials in Oncology. It enrolled patients with resected stage III colon adenocarcinoma confirmed to be MSI-H/dMMR by local or central testing. Participants were randomized 1:1 to receive:
- FOLFOX for 6 months (control arm)
- FOLFOX for 6 months plus atezolizumab (experimental arm), followed by 6 additional months of atezolizumab monotherapy
The primary endpoint was disease-free survival (DFS), with overall survival (OS) as a key secondary endpoint. The trial aimed to determine whether the addition of PD-L1 blockade to cytotoxic chemotherapy could reduce recurrence risk in this high-mutation subgroup.
Results and Key Findings
As presented at ASCO GI 2024, the ATOMIC trial enrolled approximately 750 patients between 2018 and 2022. The study did not meet its primary endpoint: the addition of atezolizumab to FOLFOX did not significantly improve DFS compared to FOLFOX alone. However, exploratory analyses revealed interesting trends.
• The 3-year DFS was not significantly different between the two arms.
• A modest benefit in DFS was seen in certain subgroups, including patients with T4 tumors or those with high nodal burden (N2 disease), though not statistically significant.
• Safety: The addition of atezolizumab was generally well tolerated, with no unexpected immune-related adverse events or additive toxicities to FOLFOX (Benson et al., 2024).
These findings contrast sharply with the efficacy of immunotherapy in metastatic MSI-H/dMMR colorectal cancer and raise important biological and clinical questions about timing, tumor microenvironment, and immune activation after resection.
Clinical Implications
The results of the ATOMIC trial underscore that adjuvant immunotherapy is not yet standard of care for stage III MSI-H/dMMR colon cancer. FOLFOX chemotherapy alone remains the primary recommendation. However, the findings suggest potential areas for further research:
- Identifying biomarkers of recurrence in MSI-H patients to guide therapy intensity
- Exploring neoadjuvant immunotherapy, as being done in the NICHE trials, which may offer better immune engagement pre-surgery
- Evaluating circulating tumor DNA (ctDNA) post-surgery to stratify recurrence risk and tailor therapy (Tie et al., 2019)
Discussion
The ATOMIC trial represents a critical effort to evaluate the role of immune checkpoint inhibitors in the adjuvant management of MSI-H/dMMR stage III colon cancer—a population with distinct molecular and immunologic features. While atezolizumab has demonstrated remarkable efficacy in the metastatic setting for MSI-H colorectal cancer, its integration into earlier-stage disease appears more complex.
The lack of significant disease-free survival (DFS) benefit observed in this study may reflect fundamental differences in tumor biology and immune dynamics in the adjuvant setting. After complete surgical resection, the residual tumor burden is minimal, potentially limiting the immunogenic stimulus necessary for checkpoint inhibitors to be effective. This contrasts with metastatic disease, where ongoing antigen presentation sustains immune activation. Furthermore, adjuvant FOLFOX may impact the immune microenvironment in ways that are not fully understood—potentially modulating or even dampening the immune response elicited by anti–PD-L1 therapy.
Despite the overall negative primary endpoint, several exploratory signals merit further investigation. Subgroup trends suggesting potential benefit in patients with higher-risk features—such as T4 or N2 disease—indicate that immunotherapy may still have a role in selected patients. In addition, the safety profile of atezolizumab in combination with chemotherapy was manageable and consistent with prior data, affirming that the regimen is tolerable even in the curative-intent setting.
The findings from ATOMIC also reinforce a broader shift in the field toward neoadjuvant rather than adjuvant immunotherapy. Early-phase trials such as NICHE have shown that immune checkpoint inhibitors administered before resection can generate robust pathological responses in MSI-H tumors. This may be due to the presence of intact tumor architecture and antigens, which are essential for effective T-cell priming. Ongoing trials are directly comparing neoadjuvant versus adjuvant immunotherapy in early-stage colorectal cancer and will be key to refining treatment sequencing.
Finally, the trial highlights the urgent need for better tools to individualize adjuvant therapy. Emerging data on circulating tumor DNA (ctDNA) suggest that molecular residual disease detection may outperform clinicopathologic risk models in identifying patients at highest risk of relapse. In future designs, integrating ctDNA status may allow more precise selection of patients who might benefit from additional therapy such as checkpoint blockade.
In summary, while the ATOMIC trial did not change current adjuvant standards, it provided essential insight into the challenges and possibilities of immunotherapy in early-stage colon cancer. Its findings will help guide the design of future precision oncology strategies that align treatment intensity with molecular risk.
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
