Paolo Tarantino, 2025 Yvonne’s “Top Voice” Award Winner, Research Fellow at Dana-Farber Cancer Institute, shared a post on LinkedIn:
“ADCs are rapidly improving the way we treat every type of cancer.
Their potential has exponentially grown over the past 6 years – yet their technology is the result of a 100-year long, epic journey.
Here’s a list of 10 key milestones in the development of ADCs – curated by Raffaele Colombo and myself.
1. MAGIC BULLET
In 1907, inspired by von Weber’s opera “Der Freyschütz”, Paul Ehrlich envisioned an ideal drug that, like the magic bullet in the opera, would hit its target without collateral damage. His vision laid the foundation for ADCs
2. FIRST CHEMO
After WW1, when researchers discovered that mustard gas, used in chemical warfare, destroyed lymphatic tissue and bone marrow, they thought it might be able to kill cancer cells in the lymph nodes.
At Yale, Goodman and Gilman began to study the effects of nitrogen mustard on lymphoma.
The trial, which started in 1942 and enrolled 67 patients, remained a U.S. military secret until 1946.
3. THE ANTIBODY
In 1975, the breakthrough in antibody therapeutics began with the development of hybridoma technology by Köhler and Milstein, enabling the production of monoclonal antibodies.
4. FIRST ADC TRIAL
In 1983, the very first clinical trial with an ADC was published:
– anti-CEA sheep antibody
– vinca payload
– non-cleavable linker
– stochastic lysine conjugation
– DAR=4 to DAR=11
5. FIRST ADC APPROVAL
In May 2000, the US FDA granted accelerated approval for the first ADC, gemtuzumab ozogamicin (Mylotarg), for patients aged 60 and older with CD33-positive relapsed AML.
6. FIRST ADC FOR A SOLID TUMOR
2013: ADCs finally improve outcomes for solid tumors. T-DM1 earns FDA approval for HER2+ metastatic breast cancer, after demonstrating improved outcomes in the EMILIA trial.
7. RISE OF TOPO-1 ADCs
From 2016 onwards, the ADC game changed with topoisomerase I inhibitors. Enter T-DXd, SG, Dato-DXd and beyond— ready to revolutionize the entire field of oncology, approval after approval.
8. FIRST HISTOLOGY-AGNOSTIC APPROVAL
2023: The FDA grants the first ever histology-agnostic ADC approval to T-DXd for HER2+ solid tumors, based on the results of DESTINY-PanTumor02.
9. ADC COMBINATIONS COME OF AGE
More and more combinations demonstrate to further enhance the efficacy of ADCs.
2023: EV + pembro doubles OS in urothelial cancer.
2024: DAD trial: first trial combining two different ADCs (EV+SG) is published
2025: Two ADC combos, SG/pembro and T-DXd/pertuzumab, reshape first-line treatment standards in breast oncology
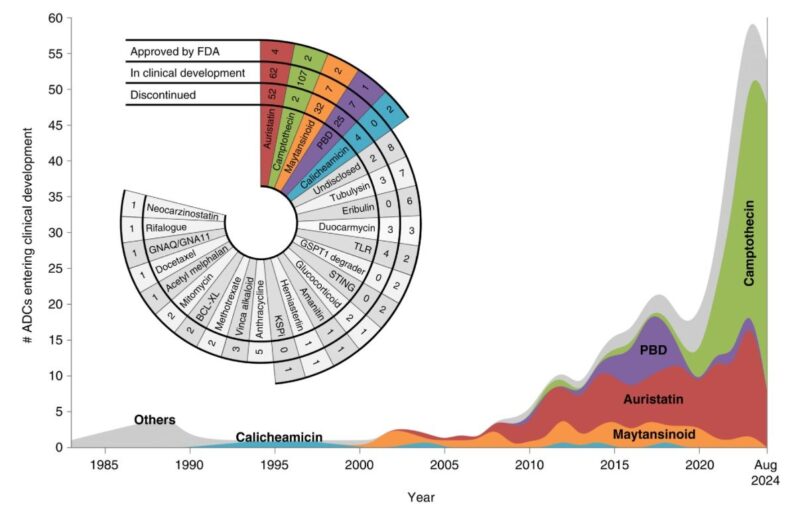
10. THE ADC WAVE
To date, over 270 ADCs have entered clinical development. Revenue from approved ADCs and those in phase III development is forecasted to reach $26 billion in 2028.
The prognosis of thousands of cancer patients has already been reshaped by ADCs, and trials of ADCs have been started for conditions beyond cancer.
The ADC era has just started.”
Title: The Journey of Antibody–Drug Conjugates: Lessons Learned from 40 Years of Development
Authors: Raffaele Colombo, Paolo Tarantino, Jamie R. Rich, Patricia M. LoRusso, Elisabeth G.E. de Vries

More posts featuring Paolo Tarantino and Raffaele Colombo on OncoDaily.


