
FDA approval of Taletrectinib for ROS1 positive Non Small Cell Lung Cancer
Taletrectinib was approved by the U.S. Food and Drug Administration (FDA) on June 11, 2025, for the treatment of adult patients with locally advanced or metastatic ROS1-positive non-small cell lung cancer (NSCLC). The approval was based on data from the phase II TRUST-I and TRUST-II studies, which demonstrated high and durable systemic and intracranial response rates.
Taletrectinib had previously received both Breakthrough Therapy and Orphan Drug Designations from the FDA, highlighting its potential to address an unmet medical need in this rare disease. ROS1-positive NSCLC represents approximately 2 percent of all NSCLC cases, or about 3,000 new diagnoses each year in the United States, and is frequently diagnosed in younger, non-smoking patients.
With its approval, taletrectinib offers a new therapeutic option alongside existing ROS1 inhibitors such as crizotinib, entrectinib, and repotrectinib, broadening the treatment landscape for patients with ROS1-positive NSCLC.
Crash course on ROS1 mutated NSCLC
Non‑small cell lung cancer (NSCLC) accounts for approximately eighty‑five percent of all lung cancer diagnoses and consists of a heterogeneous group of malignancies that grow more slowly than small cell lung cancer and tend to metastasize later in their course.
Adenocarcinoma represents the most common histologic subtype of NSCLC, arising from peripheral glandular cells and accounting for roughly forty percent of cases. This subtype frequently occurs in women and in nonsmokers and is often driven by molecular alterations such as EGFR mutations, ALK rearrangements, and KRAS mutations.
Squamous cell carcinoma, which originates from the epithelial lining of the central airways, accounts for approximately twenty‑five to thirty percent of NSCLC cases and is strongly linked to smoking; it is less likely to harbor EGFR or ALK alterations but may exhibit FGFR1 amplifications or PI3K pathway mutations.
Molecular profiling has become critical in NSCLC management, especially in adenocarcinoma, because it identifies actionable driver mutations that guide personalized targeted therapy; biomarkers such as tumor mutational burden and PD‑L1 expression are also used to predict response to immune checkpoint inhibitors
ROS1-positive non-small cell lung cancer (NSCLC) is a rare subtype that affects approximately two percent of patients with NSCLC, corresponding to about three thousand new cases diagnosed each year in the United States.
You can read the more about NSCLC here
What drug is Taletrectinib and how does it work?
Taletrectinib (Ibtrozi) is an oral tyrosine kinase inhibitor (TKI) that targets the ROS1 tyrosine kinase, a protein that can become abnormally activated in certain cancer cells. In non-small cell lung cancer (NSCLC), ROS1 gene rearrangements result in the formation of fusion proteins that drive constitutive activation of the ROS1 kinase domain.
This leads to persistent downstream signaling that promotes uncontrolled cancer cell growth, proliferation, and survival. Taletrectinib is designed to specifically inhibit these aberrantly activated ROS1 kinases, thereby interrupting the signaling pathways that fuel tumor progression.
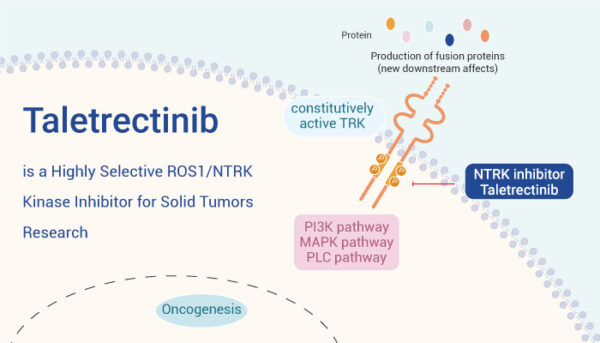
Source: cancer research network
Taletrectinib belongs to the class of tyrosine kinase inhibitors, which are drugs that block the enzymatic activity of tyrosine kinases, critical mediators of intracellular signaling. By selectively binding to the ROS1 kinase, taletrectinib prevents the transmission of growth-promoting signals, thereby inhibiting the proliferation of ROS1-rearranged cancer cells.
A key advantage of taletrectinib is its ability to effectively penetrate the central nervous system (CNS), making it an important treatment option for patients with brain metastases, a frequent complication in ROS1-positive NSCLC. Clinical trial data and pharmacologic studies have demonstrated that taletrectinib maintains robust activity within the CNS, addressing an important unmet need for intracranial disease control.
Current treatment of ROS1 positive NSCLC
There is no single global standard of care yet fully established for ROS1-mutated (ROS1-positive) NSCLC, but ROS1 tyrosine kinase inhibitors (TKIs) are the internationally recommended first-line treatment for patients with advanced disease.
Current options include:
- Crizotinib was the first approved ROS1 TKI and remains widely used, especially in settings where access to newer drugs is limited.
- Entrectinib is also approved, with better CNS penetration than crizotinib, making it preferred for patients with brain metastases.
- Repotrectinib is the most recent FDA-approved option (2023), designed for both systemic and intracranial activity and to overcome common resistance mutations like ROS1 G2032R. Many experts now consider repotrectinib a preferred agent for newly diagnosed ROS1-positive NSCLC when available.
- Taletrectinib (Ibtrozi), just FDA-approved (June 2025), is expected to become another preferred treatment option based on strong efficacy, CNS activity, and favorable tolerability seen in TRUST-I and TRUST-II trials.
Preliminary studies of Taletrectinib
Preliminary clinical evaluation of taletrectinib was conducted in the phase II TRUST-I and TRUST-II studies, which provided the foundational efficacy and safety data supporting its regulatory approval for the treatment of ROS1-positive NSCLC.
A total of 273 ROS1‑positive patients received taletrectinib 600 mg once daily: 160 TKI‑naïve and 113 pretreated with prior ROS1 TKIs. Patients also received prior chemotherapy based on clinician discretion. Key subgroups included
- Race: Asian vs. non‑Asian
- Region: Western vs. non‑Western
- Prior chemotherapy: yes vs. no
Endpoints
Primary endpoint was confirmed objective response rate (cORR) by blinded independent central review per RECIST v1.1.
Secondary endpoints were duration of response (DOR), disease control rate (DCR), progression‑free survival (PFS), intracranial efficacy, and safety outcomes—including treatment-emergent adverse events (TEAEs; graded per CTCAE).
Study Design
Both TRUST‑I (China) and TRUST‑II (global) are multicenter, single-arm, open-label phase II trials evaluating taletrectinib in locally advanced or metastatic ROS1‑positive NSCLC. The pooled analysis (Abstract 503076) assessed consistency in efficacy and safety across the two trials and across patient subgroups defined by race, region, prior TKI exposure, and chemotherapy history
Results
TRUST-I and TRUST-II trials demonstrated high and durable systemic and intracranial responses in patients with ROS1-positive NSCLC. The integrated analysis of these studies was presented as poster presentations at the 2025 American Society of Clinical Oncology (ASCO) Annual Meeting, underscoring the global importance of these findings and supporting the use of taletrectinib as a new standard option in this rare molecular subtype.
Efficacy in TKI‑Naïve Patients
- cORR was 88.8% overall (95% CI 82.8–93.2%).
- Complete response (CR) in 8 patients; partial response in 134.
- DCR reached 95.0%.
- Median DOR extended to 44.2 months (95% CI 30.4–NR).
- Median PFS was 45.6 months (95% CI 29.0–NR); 12‑month PFS rate was 39.7%.
- Intracranial cORR was 76.5%; median intracranial DOR was 14.7 months
Efficacy in TKI‑Pretreated Patients
- cORR was 55.8% (95% CI ~52–62% across trials).
- Median systemic DOR was 16.6 months; median PFS was 9.7 months.
- Intracranial cORR stood at 65.6%; intracranial DOR was 11.9 months
Subgroup Analyses
- Race (Asian vs non‑Asian) and region (Western vs non‑Western) showed comparable cORRs in both TKI‑naïve and pretreated groups.
- Prior chemotherapy status had minimal effect on efficacy—high response rates observed regardless of chemotherapy exposure
Safety
- Overall grade ≥3 TEAE rates were 54.3% (TRUST‑I) vs 52.6% (TRUST‑II).
- Grade ≥3 liver enzyme elevations: AST ~20%, ALT ~18–20%.
- Grade ≥3 GI disorders: 5.8% vs 4.1% (TRUST‑I vs II).
- Common all‑grade TEAEs included diarrhea (56–64%), nausea (47–52%), vomiting (33–43%), dizziness (~17–22%), rash (22%), and fatigue (~20%).
- Most CNS effects were grade 1–2, transient; dose reductions occurred in ~5%, and discontinuations due to TEAEs were ~6–7%
Key Findings
- Robust systemic efficacy: ~89% response in TKI‑naïve; ~56% in pretreated patients.
- Durable benefit: median DOR of 44.2 months (naïve) and 16.6 months (pretreated).
- Strong intracranial activity: ~76% intracranial response in treatment‑naïve; ~66% in pretreated.
- Consistent across demographics: Similar efficacy and safety across race (Asian vs non‑Asian) and region.
- Manageable safety profile: Grade ≥3 TEAEs common but largely reversible; low discontinuation (~6–7%).
Key Take‑Away Messages
- Taletrectinib offers best‑in‑class systemic and CNS efficacy in ROS1‑positive NSCLC with durable responses.
- The drug’s efficacy is maintained across diverse patient populations—TKI‑naïve, pretreated, Asian, non‑Asian, Western, non‑Western.
- The safety profile is acceptable, with predictable hepatic and GI AEs and minimal treatment discontinuations.
- Brain metastases, a major challenge in ROS1‑rearranged NSCLC, responded in ~70% of evaluable patients.
These results support taletrectinib’s role as a frontline or subsequent ROS1 TKI, pending further randomized confirmation.
You can read the full article here.
Written by Sona Karamyan, MD
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
