VAYHIT-2 trial results were presented during the OncoNext media briefing, offering important new evidence for the use of the monoclonal antibody ianalumab in combination with eltrombopag for adults with primary immune thrombocytopenia (ITP) who failed first-line corticosteroid therapy. The findings highlight the potential of a short, time-limited course of B-cell–directed therapy to achieve durable platelet responses without the need for ongoing treatment—addressing a long-standing gap in ITP management.
Background
Primary ITP is a rare autoimmune disorder in which the immune system mistakenly targets and destroys platelets, the cells that are essential for blood clotting. Patients may experience prolonged bleeding, easy bruising, and significant fatigue, with symptoms that can affect daily functioning and quality of life. Many individuals cycle through several therapies in search of sustainable disease control, and currently approved second-line treatments often require long-term or indefinite use. Attempts to shorten treatment duration have had limited success, underscoring the need for therapies with novel mechanisms that deliver durable responses while reducing treatment burden.
Ianalumab is a fully human monoclonal antibody designed to target B cells through a dual mechanism of action. The drug promotes B-cell depletion via antibody-dependent cellular cytotoxicity and simultaneously inhibits BAFF-receptor signaling, which is critical for the activation and survival of autoreactive B cells. By disrupting these key steps in the autoimmune cascade, ianalumab may help restore immune regulation and reduce platelet destruction. Previous studies suggested promising activity in later-line ITP and provided the rationale to explore earlier use.
VAYHIT2 Trial Design
The VAYHIT2 study enrolled adults with primary ITP who had been treated only with corticosteroids, with or without intravenous immunoglobulin, and who had either an insufficient response or a relapse. At enrollment, all participants had platelet counts below 30 × 10⁹/L, had not received any second-line therapy, and were candidates to initiate eltrombopag.
Patients were randomized in a 1:1:1 ratio to receive
- ianalumab 3 mg/kg
- ianalumab 9 mg/kg
- placebo.
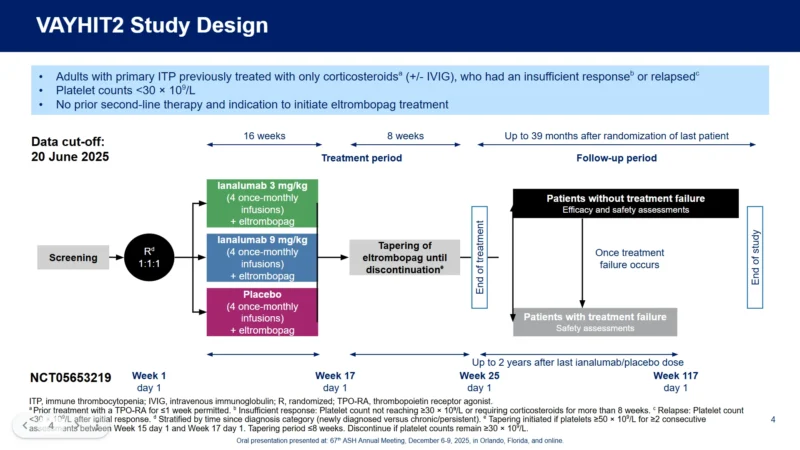
Each treatment arm received four once-monthly infusions combined with daily eltrombopag for the initial 16 weeks. Starting at week 16, eltrombopag was tapered and discontinued over an 8-week period if platelet responses were adequate. Patients entered long-term follow-up and were monitored for up to 39 months after randomization—the most extended prospective evaluation to date for early-line B-cell–directed therapy in ITP.
The primary endpoint was time to treatment failure, a clinically relevant composite that included platelet count decline, bleeding events, the need for rescue therapy, hospitalization, or recurrent need for ITP treatment. The key secondary endpoint was stable platelet response at six months (SR6).
Results
VAYHIT2 met its primary endpoint, demonstrating that both doses of ianalumab significantly prolonged time to treatment failure compared with placebo. The 9 mg/kg dose yielded a hazard ratio of 0.55 (95% CI 0.32–0.92; p=0.021), while the 3 mg/kg dose demonstrated a hazard ratio of 0.58 (95% CI 0.34–0.98; p=0.023). The median time to treatment failure was 13.0 months for the 9 mg/kg arm and was not reached for the 3 mg/kg arm, compared with 4.7 months in the placebo group. These results suggest that a short course of ianalumab in combination with eltrombopag can sustain disease control long after active treatment ends.
Key Findings of VAYHIT-2 Trial
TTF was longer with both ianalumab doses compared with placebo:
- 9 mg/kg: HR = 0.55 (95% CI 0.32–0.92), p = 0.021
- 3 mg/kg: HR = 0.58 (95% CI 0.34–0.98), p = 0.023
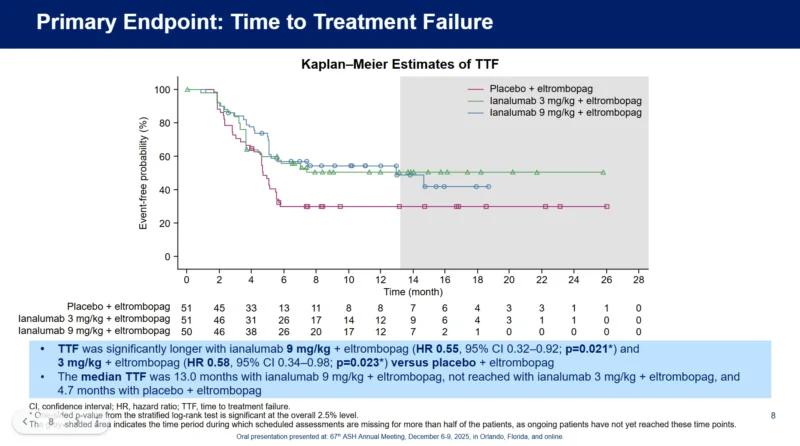
The median time to treatment failure was 13.0 months for the 9 mg/kg arm and was not reached for the 3 mg/kg arm, compared with 4.7 months in the placebo group. 13.0 months (9 mg/kg). Stable response at six months was achieved by a significantly higher proportion of patients treated with ianalumab.
The 9 mg/kg group achieved an SR6 rate of 62.0%, compared with 39.2% in the placebo group. The 3 mg/kg dose also demonstrated a numerically higher response rate of 56.9%, supporting the overall activity of both dosing strategies.
Safety Profile
Ianalumab was generally well tolerated. Adverse event rates were consistent across treatment groups, and most were mild to moderate. Transient neutropenia occurred in some patients but resolved without clinical complications, and no cases of febrile neutropenia or neutropenic sepsis were reported. Infection rates were similar across all study arms, and no new safety concerns emerged. Overall, the safety profile aligns with prior experience and supports further development in earlier lines of therapy.
Implications for the ITP Treatment Landscape
The VAYHIT2 results provide compelling evidence that a limited course of ianalumab plus eltrombopag may enable patients with primary ITP to achieve and maintain meaningful disease control without chronic therapy. This represents an important shift from the current paradigm of continuous treatment toward an approach that aims for durable, treatment-free remission. Longer-term follow-up will further clarify whether early intervention with B-cell–directed therapy may have disease-modifying effects in ITP.
Ianalumab continues to be studied across the disease spectrum, including in the phase 3 VAYHIT1 trial as a first-line therapy and in the phase 2 VAYHIT3 study in third- and later-line ITP.
For more information click here.