The Phase 1 trial of TCR-engineered T cells targeting PRAME (NCT03686124), presented by Dr. Sapna P. Patel at the ESMO Congress 2025, demonstrated remarkable clinical activity in patients with metastatic uveal melanoma (UM), a malignancy with few effective treatment options. Uveal melanoma represents approximately 5% of all melanomas, and up to half of patients eventually develop metastatic disease, for which prognosis remains poor. The study evaluated IMA203, a PRAME-directed TCR T-cell therapy, designed to recognize intracellular PRAME-derived peptides presented by HLA-A*02:01 and trigger a potent, tumor-specific immune response.
Background
Although tebentafusp is currently the only approved systemic therapy for metastatic UM, durable disease control remains rare, particularly in pretreated populations. PRAME (Preferentially Expressed Antigen in Melanoma) is expressed in about 90% of uveal melanomas and is associated with worse survival outcomes, making it an attractive target for cellular immunotherapy.
This Phase 1a/b, multicenter, dose-escalation and dose-expansion trial evaluated the safety, tolerability, pharmacodynamics, and preliminary efficacy of TCR-engineered autologous T cells expressing PRAME-specific receptors (IMA203) in patients with advanced solid tumors, including a dedicated uveal melanoma cohort.
Methods
Eligible patients were ≥18 years old, HLA-A*02:01-positive, and PRAME-positive, with recurrent or refractory solid tumors and no remaining standard-of-care options. The study included uveal melanoma patients previously treated with tebentafusp.
After leukapheresis and manufacturing of individualized TCR-modified T cells, patients received lymphodepletion (LD) with cyclophosphamide (500 mg/m² × 4 days) and fludarabine (30 mg/m² × 4 days), followed by infusion of IMA203 T cells and low-dose interleukin-2 (IL-2) support (1 million IU once daily × 5 days, then twice daily × 5 days subcutaneously).
The study assessed treatment-emergent adverse events (TEAEs), objective response rate (ORR), duration of response (DOR), progression-free survival (PFS), overall survival (OS), and T-cell persistence.
Results of PRAME-Directed TCR Therapy
As of April 7, 2025, 74 heavily pretreated patients (median 3 prior lines) were enrolled across tumor types.
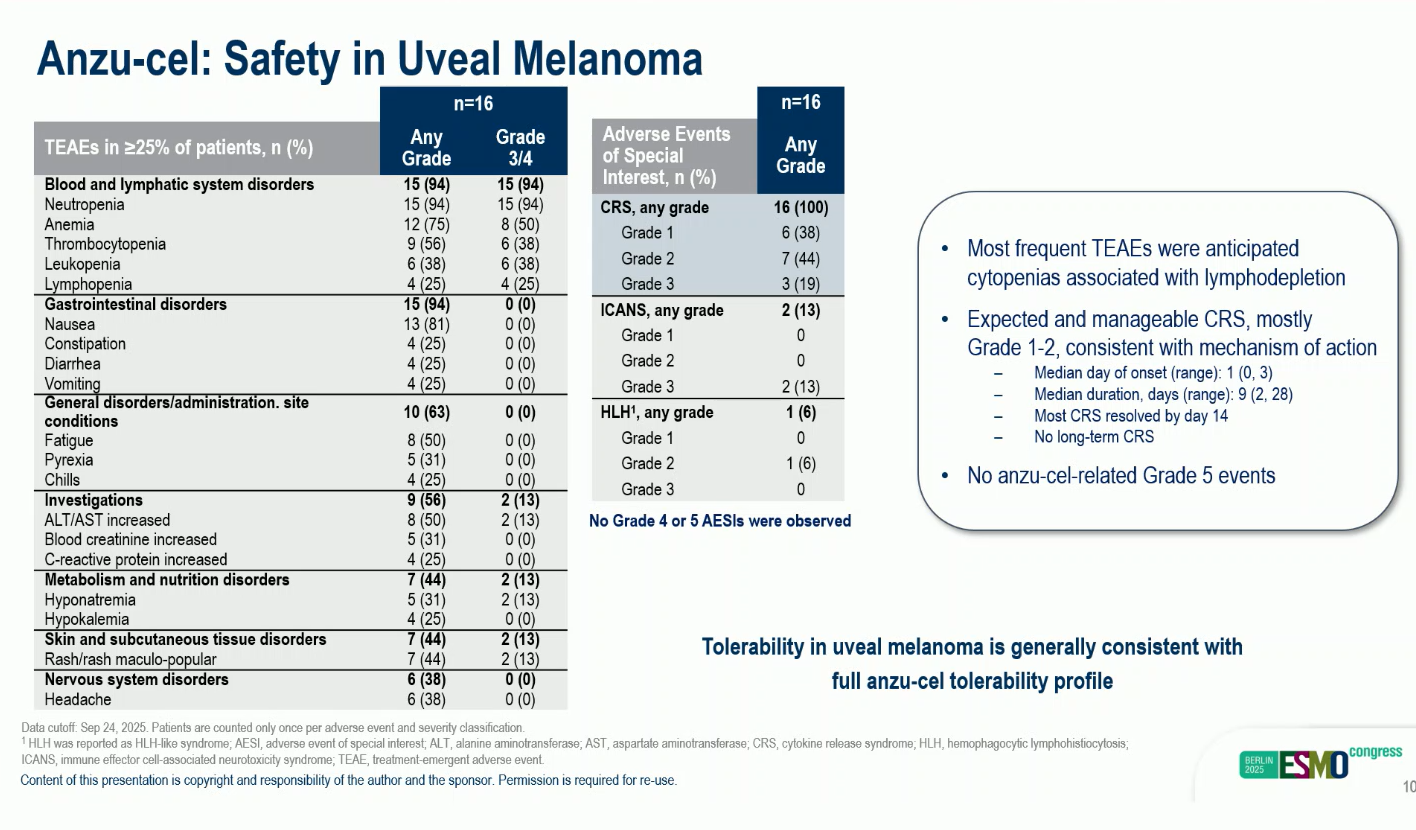
Most common TEAEs: lymphodepletion-related cytopenias (99%, Grade 3–4), cytokine release syndrome (CRS, 95%; mostly Grade 1–2, resolving by day 14), and infrequent ICANS (13%; no Grade ≥4).
In the uveal melanoma cohort (Phase 1b, n = 16):
- Median 2 prior lines of therapy; 62.5% had received tebentafusp.
- Median ORR: 69% (11/15), confirmed ORR 67%
- Tumor shrinkage: observed in all 16 patients.
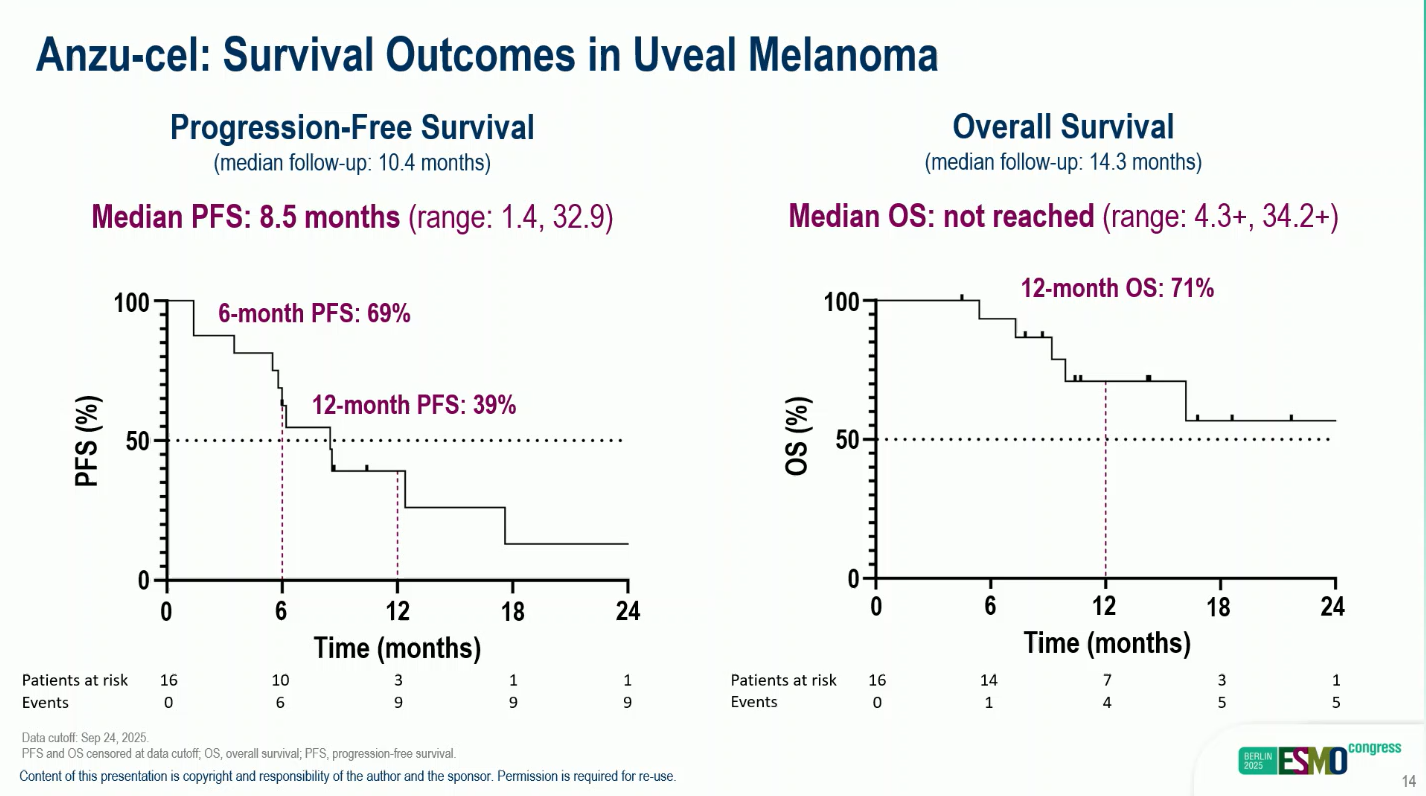
- Median DOR: 11.0 months (range 1.8+–31.6)
- Median PFS: 8.5 months (range 1.4–32.9).
- Median OS not reached, 12-mounts OS 71%
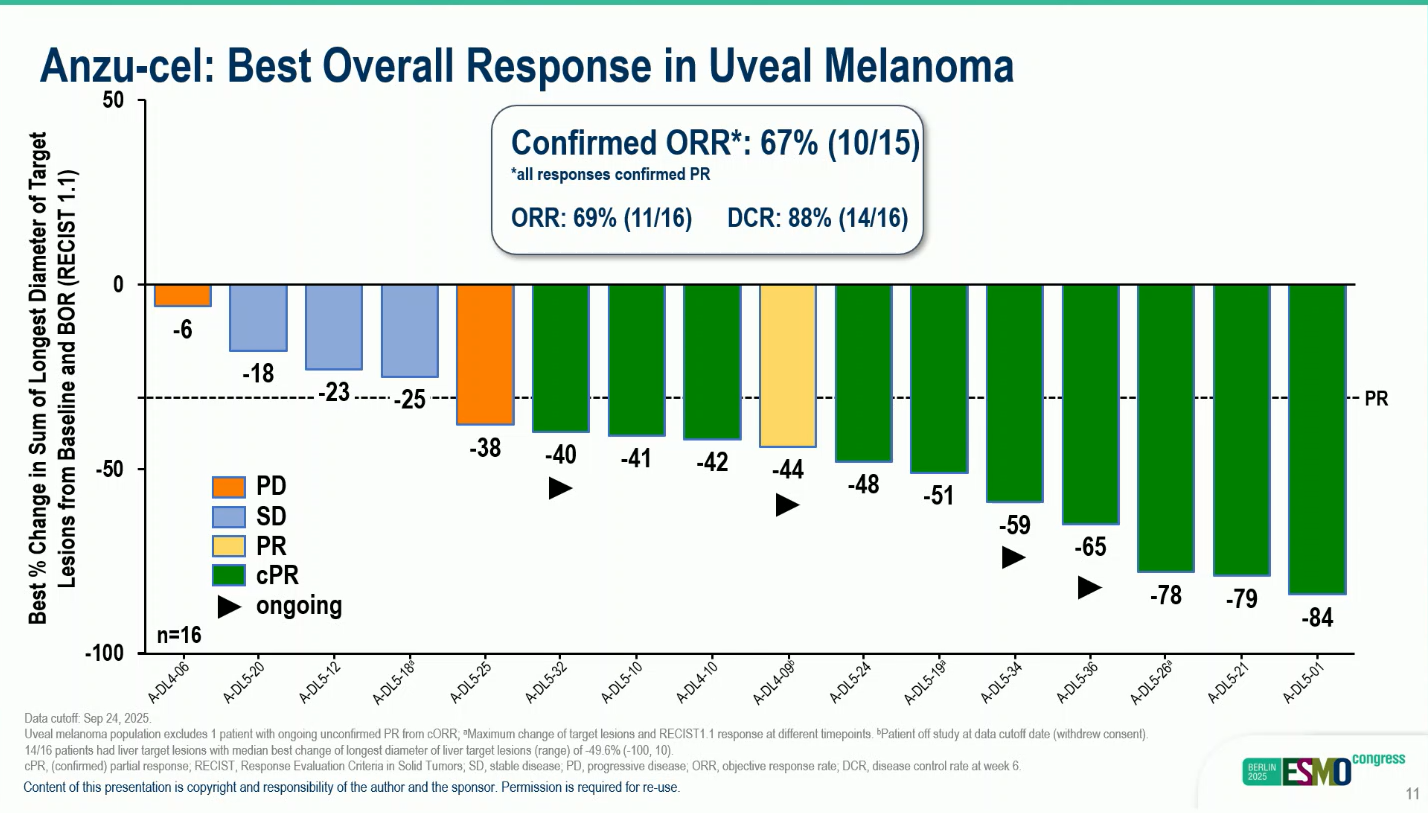
These results demonstrate strong and sustained anti-tumor activity with encouraging durability, even in patients with prior tebentafusp exposure and large-volume disease.
Conclusions
The Phase 1 PRAME-directed TCR T-cell therapy trial (NCT03686124) demonstrated notable clinical efficacy, achieving a 67% objective response rate (ORR) with durable tumor regression in patients with metastatic uveal melanoma (UM)—a cancer subtype with historically poor outcomes and limited treatment options. Responses were observed across a heavily pretreated population, including individuals who had previously received tebentafusp, reinforcing the potential of PRAME-targeted TCR therapy to overcome therapeutic resistance.
The treatment’s favorable tolerability profile further distinguishes it from many existing immunotherapies. The majority of treatment-emergent adverse events were related to lymphodepletion and cytokine release, and were predominantly low grade, transient, and manageable. Importantly, no Grade 4 cytokine release syndrome or neurotoxicity (ICANS) was reported, highlighting the therapy’s safety even in a population with advanced disease.
In addition to strong response rates, the trial demonstrated prolonged duration of response (median 11 months) and sustained T-cell persistence, with rapid engraftment and long-term in vivo activity lasting up to 900 days in some patients. This sustained presence of PRAME-specific T cells indicates durable immune surveillance, a key factor in preventing disease recurrence.
Collectively, these findings support TCR-engineered cell therapy as a breakthrough immunotherapeutic platform capable of producing deep and lasting responses in metastatic uveal melanoma. The planned phase 2 expansion aims to confirm these results in a larger cohort, refine patient selection based on PRAME expression levels and HLA-A*02:01 status, and further explore biomarkers predictive of durable benefit. If confirmed, this approach could mark a major advancement in personalized cell therapy for patients with uveal melanoma—a disease long regarded as resistant to immunotherapy.
You can read the full abstract here.