The TBCRC-056 Trial was designed to evaluate a chemotherapy-free neoadjuvant regimen combining the PARP inhibitor niraparib with the anti–PD-1 antibody dostarlimab in patients with germline BRCA1/2 or PALB2–mutated, HER2-negative breast cancer.
Germline BRCA1/2 and PALB2 mutations define a biologically distinct subset of breast cancer characterized by homologous recombination deficiency and sensitivity to PARP inhibition. PARP inhibitors have an established role in the management of germline BRCA-mutated breast cancer, and when used as neoadjuvant monotherapy, pathologic complete response (pCR) rates approaching 50% have been reported in triple-negative breast cancer (TNBC).
Preclinical data further suggest synergy between PARP inhibition and immunotherapy, with PARP inhibitors activating the cGAS–STING pathway, promoting CD8+ T-cell recruitment and potentially sensitizing tumors to immune checkpoint blockade. While combinations of PARP inhibitors and anti–PD-1/PD-L1 agents have not improved outcomes in the metastatic setting, this strategy may have greater impact in earlier, less immunosuppressed disease.
Study Design and Methods
TBCRC-056 is a prospective, multicenter, investigator-initiated phase II study with three arms. Arms A and B enrolled patients with stage I–III TNBC (tumor size ≥1.0 cm, ER <10%, HER2-negative) harboring germline BRCA1/2 or PALB2 mutations, while Arm C was an exploratory cohort for ER-positive disease.
Patients in the TNBC cohorts were randomized to one of two treatment strategies.
- Arm A received upfront niraparib plus dostarlimab for 18 weeks.
- Arm B received a 3-week lead-in of niraparib monotherapy followed by the addition of dostarlimab for a total of 15 weeks.
Niraparib was administered orally at 200 mg daily, and dostarlimab was given intravenously at 500 mg every three weeks. After completion of neoadjuvant therapy, patients proceeded to surgery or crossed over to receive additional preoperative systemic therapy if indicated. Mandatory tumor biopsies were obtained at baseline and at cycle 2 day 1 to assess stromal tumor-infiltrating lymphocytes (sTILs).
The primary objectives were to evaluate the pCR rate with preoperative niraparib and dostarlimab and to assess changes in stromal TILs from baseline to cycle 2.
Patient Characteristics and Treatment Exposure
A total of 46 patients with TNBC were enrolled into Arms A and B between January 2021 and February 2025 across eight TBCRC sites. The median age was 39 years, with approximately half of patients presenting with stage II disease and 24% having node-positive disease. Germline BRCA1 mutations were present in 83% of patients, and BRCA2 mutations in 17%.
Treatment exposure was high, with 82.6% of patients completing the target number of cycles of both dostarlimab and niraparib. The mean number of cycles received was 5.1 for dostarlimab and 5.7 for niraparib. Eleven patients (23.9%) received additional neoadjuvant therapy prior to surgery.
Results
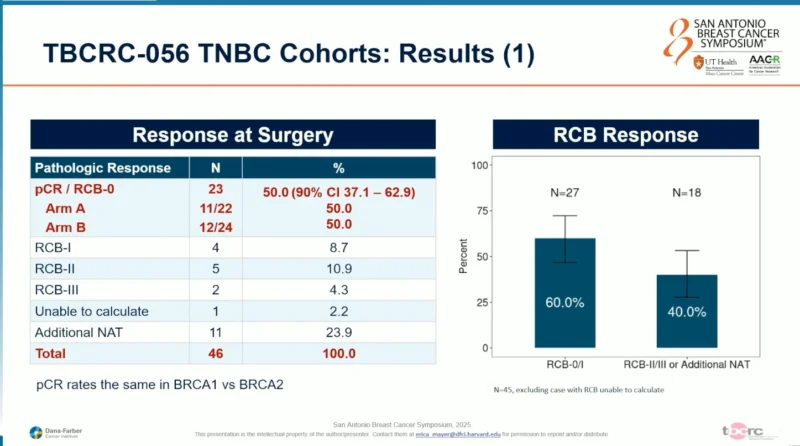
At surgery, 23 of 46 patients (50%) achieved a pCR (RCB-0), meeting the primary efficacy endpoint of the study. pCR rates were identical in Arms A and B. When residual cancer burden was evaluated, an RCB-0/I rate of 60% was observed, with RCB-II or III in 40% of patients. pCR rates were similar between patients with BRCA1 and BRCA2 mutations.
Immune Correlates and sTILs Analysis
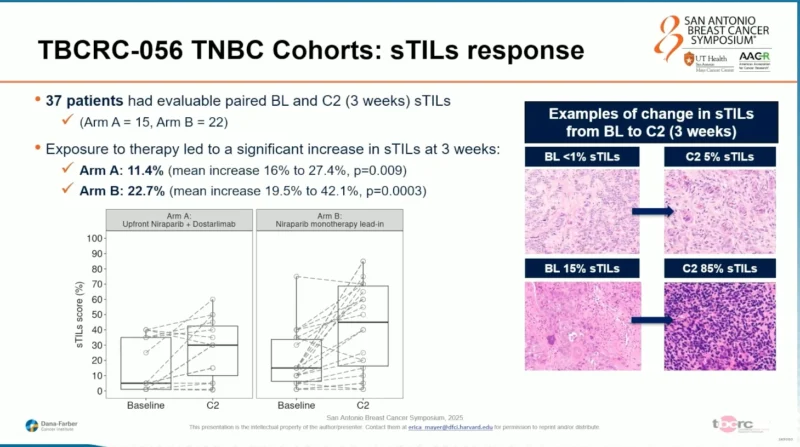
Thirty-seven patients had evaluable paired baseline and cycle 2 biopsies for stromal TIL assessment. Exposure to therapy led to a significant increase in sTILs at 3 weeks, fulfilling the second primary endpoint. The mean increase in sTILs was 11.4% in Arm A (upfront combination; p=0.009) and 22.7% in Arm B (niraparib lead-in; p=0.0003). Representative histologic examples demonstrated both modest and marked increases in immune infiltration, with some tumors increasing from <1% to 5% sTILs and others from 15% to 85%.
Baseline sTILs were evaluable in 45 patients, with a median baseline score of 10%. When analyzed as a continuous variable, higher baseline sTILs were significantly associated with pCR or RCB-0/I at surgery. In contrast, change in sTILs from baseline to cycle 2, baseline PD-L1 expression, and baseline ER status were not associated with pathologic response.
The safety profile was consistent with the known toxicities of niraparib and dostarlimab. Six patients (13%) discontinued all protocol therapy early, including three due to toxicity and three due to inadequate response or disease progression. Treatment-related adverse events occurring in ≥10% of patients included anemia, fatigue, hypertension, hypothyroidism, neutropenia, rash, and headache. Grade 3 or higher events were infrequent, and one grade 4 neutropenia was reported.
Conclusions and Clinical Implications
In patients with germline BRCA-mutated stage I–III TNBC, 18 weeks of a non-chemotherapy neoadjuvant regimen with niraparib plus dostarlimab, with or without a PARP inhibitor lead-in, met both primary endpoints of the TBCRC-056 study. The regimen achieved a pCR rate of 50%, an RCB-0/I rate of 60%, and induced early increases in stromal TILs after one cycle of therapy. The contribution of immunotherapy beyond PARP inhibition in this setting remains unclear. Higher baseline sTILs were associated with pathologic response, and ongoing correlative studies aim to further define the tumor microenvironment changes associated with this approach.
For more information click here.


