CAR-T cell therapy has rapidly transitioned from breakthrough clinical trials to an established therapeutic modality across multiple B-cell malignancies, confirming its capacity to induce deep and durable remissions in otherwise refractory disease. Yet real-world experience has revealed persistent structural and biological barriers: toxicity profiles that restrict eligibility and demand intensive monitoring, manufacturing timelines that delay treatment or prevent infusion entirely, relapse driven by antigen escape and microenvironmental resistance, and profound inequities in access linked to infrastructure and referral pathways.
At Tandem 2026, the field’s focus appeared to shift decisively away from the traditional question of which antigen to target toward the more consequential challenge of how cellular therapies are engineered, delivered, and individualized. Three themes emerged with particular relevance for clinicians and trialists:
- Biomimetic receptor engineering aimed at widening the therapeutic index
- Allogeneic CAR-T strategies demonstrating early clinical feasibility
- Histology-specific dosing and toxicity patterns challenging cross-disease extrapolation
Together, these developments signal the beginning of a platform-driven era in cellular therapy.
Theme 1 — Biomimetic Design and Dual Targeting to Widen the Therapeutic Index
A central paradox of CAR-T therapy persists: the same immune activation responsible for tumor eradication also drives inflammatory toxicity. Current mitigation strategies—IL-6 blockade for cytokine release syndrome and corticosteroids for severe neurotoxicity—remain largely reactive rather than preventive, and even low-grade toxicity frequently necessitates hospitalization and resource-intensive monitoring.
Tandem 2026 highlighted an emerging design-first philosophy: instead of managing toxicity after it occurs, receptor architecture itself may be engineered to regulate signaling intensity and reduce uncontrolled immune amplification.
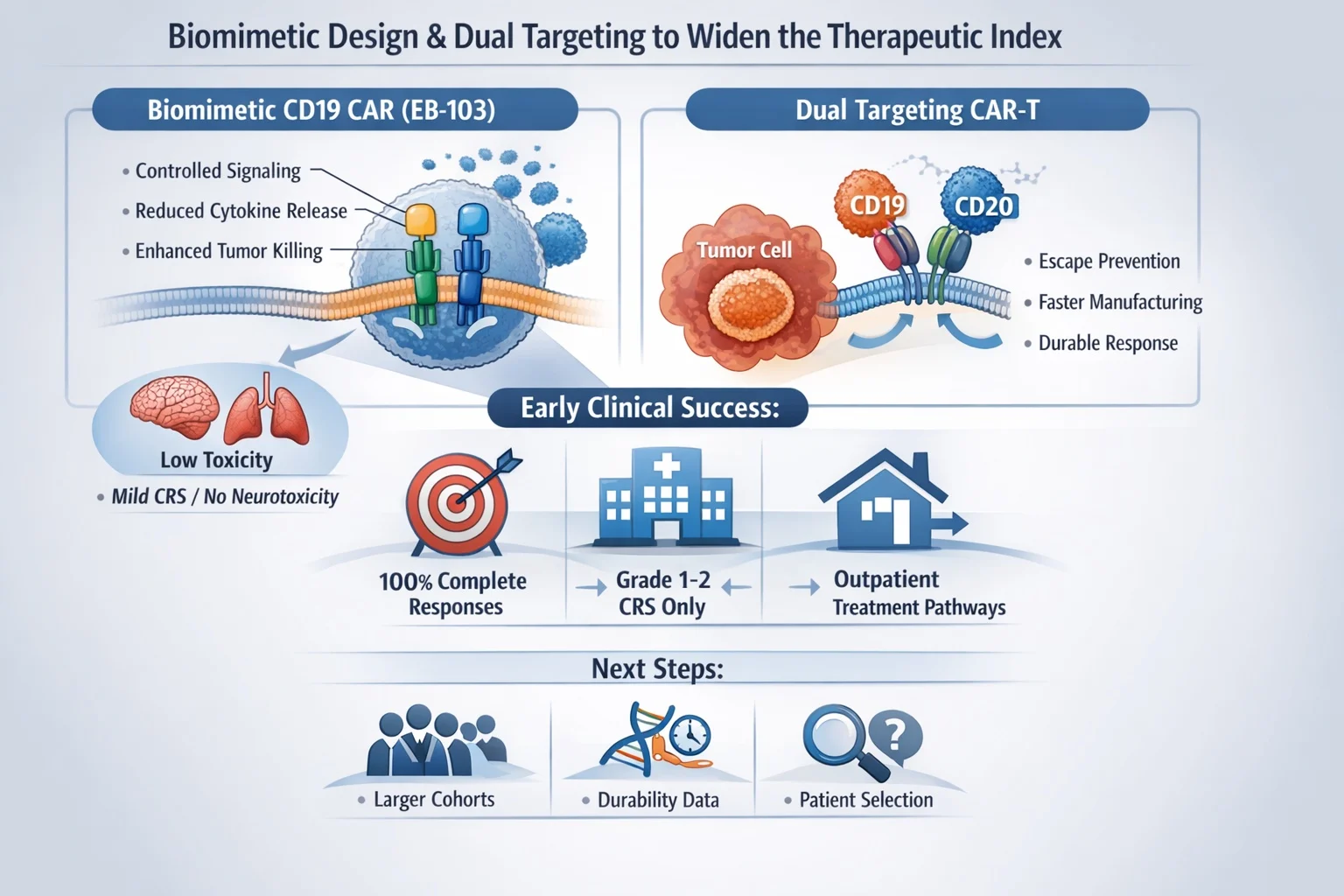
Biomimetic CD19 Engineering
The biomimetic CD19 construct EB-103 (ARTEMIS platform) illustrated this principle. By separating activation and costimulatory signaling into distinct components—mirroring physiologic T-cell signaling—the platform aims to minimize tonic signaling and cytokine excess while preserving antitumor potency.
Early clinical experience reported:
- 100% objective and complete response rates in aggressive B-cell lymphoma
- Only grade 1–2 CRS, with no high-grade inflammatory toxicity
Although based on a small cohort, responses across high-risk disease features—including bulky tumors, older patients, and CNS involvement—make the safety signal particularly provocative. If validated, such architectures could expand eligibility, reduce ICU-level complications, and enable outpatient treatment pathways.
Dual Targeting and Manufacturing Speed
Dual-target CD19/CD20 constructs further emphasized that platform design influences not only antigen escape but also cytokine biology, persistence, and deliverability. Shortened manufacturing timelines observed in early studies may preserve less-differentiated T-cell phenotypes associated with durability while reducing attrition during bridging therapy.
For clinicians, manufacturing speed is no longer logistical—it is biological and prognostic.
Clinical Interpretation
These early signals suggest receptor engineering can meaningfully reshape the toxicity-efficacy balance.
However, practice-changing conclusions require:
- Larger cohorts
- Durability data
- Identification of patient subgroups most likely to benefit
Theme 2 — Allogeneic CAR-T and the Path Toward Practical Availability
Limited access remains one of the most consequential constraints of autologous CAR-T therapy. Delays in referral, apheresis failure, and manufacturing time frequently prevent treatment altogether.
Allogeneic CAR-T products promise:
- Immediate availability
- Standardized manufacturing
- Potentially broader real-world access
Historically, however, host rejection and graft-versus-host disease have limited feasibility.
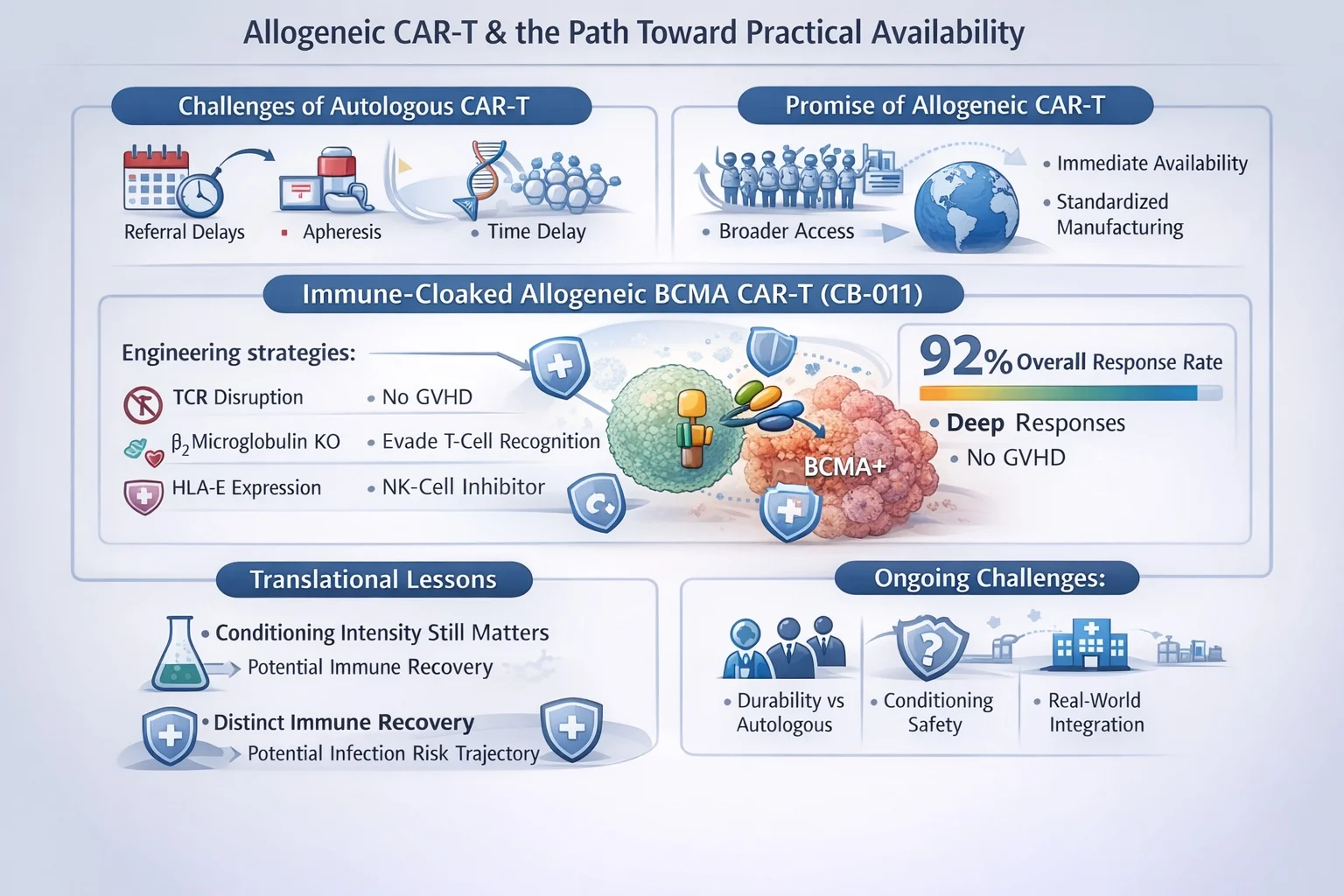
Immune-Cloaked Allogeneic BCMA CAR-T
The CB-011 program demonstrated how multilayer immune-evasion engineering may overcome these barriers. Reported findings included:
- 92% overall response rate
- Deep MRD-negative responses
- No observed GVHD
Engineering strategies combined:
- TCR disruption to prevent GVHD
- β2-microglobulin disruption to evade host T-cell recognition
- HLA-E expression to inhibit NK-cell–mediated clearance
This multi-constraint approach reflects a new paradigm: solving one immune barrier is insufficient—systems-level immune compatibility is required.
Translational Lessons
Two clinically important insights emerged:
- Conditioning intensity still matters.
Reduced lymphodepletion limited expansion and efficacy, whereas intensified conditioning restored activity—indicating persistent immune rejection pressures despite cloaking. - Immune recovery may differ from autologous CAR-T.
Relatively rapid host immune reconstitution suggests a potentially distinct infection-risk trajectory compared with continuous bispecific therapies.
Clinical Interpretation
Allogeneic CAR-T is approaching feasibility but key uncertainties remain:
- Durability versus autologous benchmarks
- Safety of intensified conditioning in broader populations
- Sequencing after prior BCMA exposure
- Real-world integration and access
These are as much implementation questions as biological ones.
Theme 3 — Histology-Specific Dosing and Toxicity Beyond DLBCL Extrapolation
Much of early CAR-T development was guided by experience in diffuse large B-cell lymphoma, which naturally led to the assumption that dosing strategies and toxicity management could be broadly extrapolated across B-cell malignancies. Insights presented at Tandem 2026 challenge this assumption and instead emphasize that underlying disease biology can meaningfully reshape both toxicity phenotype and optimal therapeutic intensity.
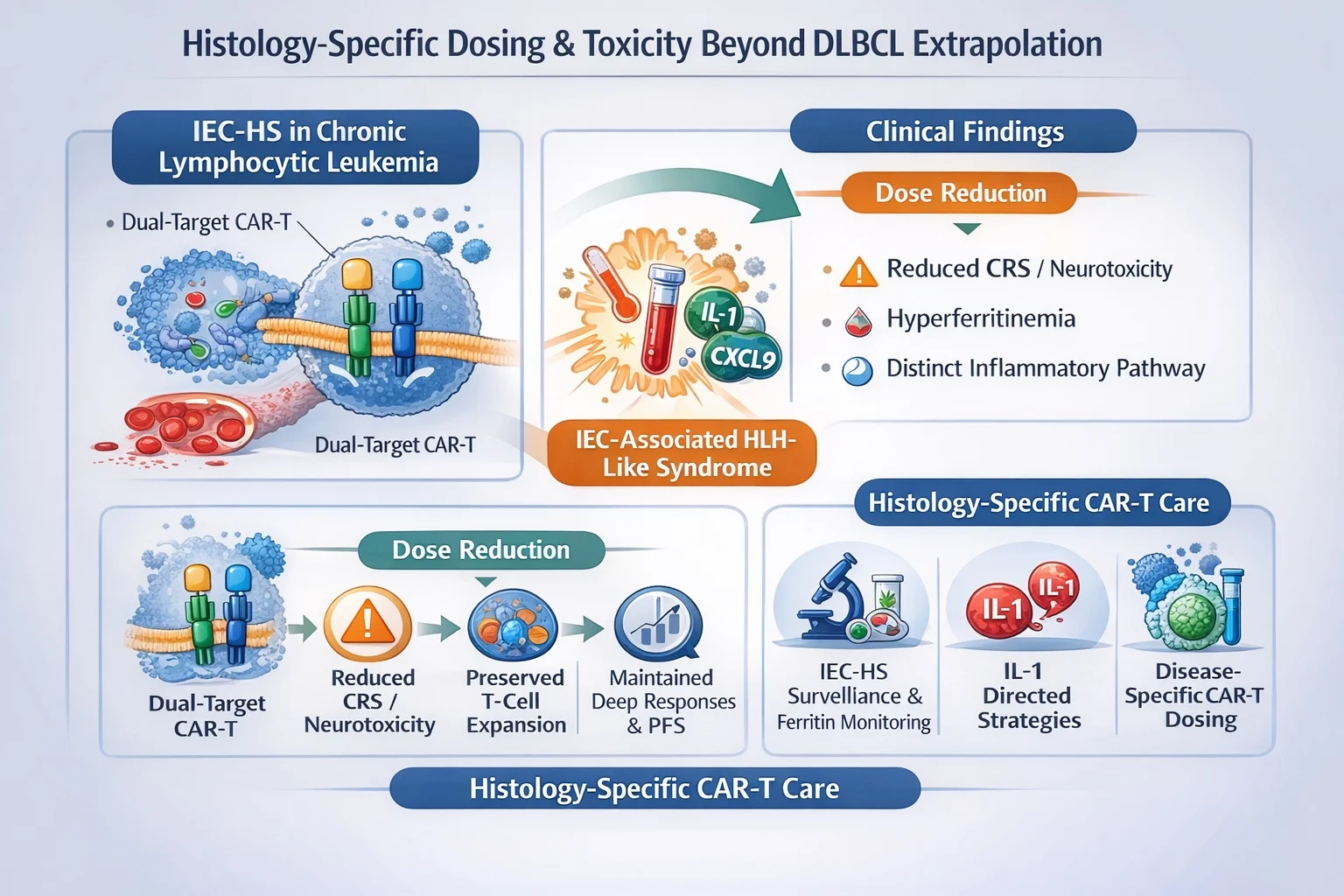
IEC-HS in Chronic Lymphocytic Leukemia
Data from dual-target CAR-T therapy in chronic lymphocytic leukemia illustrate this shift particularly clearly. In this setting, investigators observed an unexpectedly high incidence—approaching 50 percent—of immune effector cell–associated HLH-like syndrome (IEC-HS). This inflammatory syndrome was characterized by marked hyperferritinemia and cytokine patterns distinct from classical IL-6–driven cytokine release syndrome, suggesting a biologically different immune activation pathway rather than a simple extension of known CRS mechanisms.
Importantly, dose reduction proved clinically informative rather than detrimental. Lowering the administered cell dose reduced rates of severe CRS and neurotoxicity while preserving cellular expansion, maintaining deep responses, and supporting encouraging early progression-free survival signals. These findings imply that the marrow-dominant and circulating disease biology typical of CLL may interact uniquely with myeloid inflammatory circuitry, thereby requiring disease-specific dosing strategies and monitoring frameworks rather than reliance on lymphoma-derived paradigms.
Clinical Interpretation
Taken together, these observations indicate that future CAR-T practice must evolve toward histology-aware toxicity management. This includes:
- Prospective surveillance for IEC-HS rather than retrospective recognition
- Integration of ferritin kinetics and broader inflammatory biomarkers into routine monitoring
- Consideration of IL-1–directed therapeutic strategies when inflammatory biology diverges from classical CRS
What once appeared to be subtle biological nuance is rapidly becoming a practical clinical requirement. Histology-tailored toxicity frameworks are no longer academic refinements—they are likely to shape safe and effective CAR-T delivery across diseases.
Cross-Cutting Questions: Speed, Durability, and Implementation
Beyond disease-specific toxicity, Tandem 2026 highlighted three broader determinants that will ultimately define the real-world impact of next-generation cellular therapy.
Manufacturing speed is increasingly recognized as a core therapeutic variable rather than a logistical detail. Faster production timelines may preserve favorable T-cell phenotypes, reduce the need for intensive bridging therapy, and lower the risk that patients clinically deteriorate before infusion.
Durability of response remains the most critical unanswered question. Many current datasets are early-phase with limited follow-up, leaving uncertainty regarding long-term disease control across emerging platforms.
Real-world scalability will depend not only on biological innovation but also on outpatient feasibility, infrastructure readiness, reimbursement pathways, and equitable global distribution. Engineering progress alone cannot deliver population-level impact without parallel system-level evolution.
Limitations of Current Evidence
Interpretation of these advances must remain grounded in the realities of the available data. Most signals arise from early-phase studies with small patient cohorts and limited follow-up, frequently using single-arm designs that restrict comparative conclusions. In addition, enrolled populations may not fully reflect broader community practice, introducing potential selection bias.
These limitations should not diminish the importance of the observed signals. Rather, they define the next research agenda—larger expansion cohorts, longer follow-up, and rigorous comparative evaluation—required to translate early promise into durable clinical standards.
Conclusion
- Cellular therapy is entering a platform-driven era, where receptor engineering, immune compatibility, and delivery logistics may matter as much as antigen selection.
- Histology-dependent toxicity biology is now evident, particularly IEC-HS in CLL, underscoring the need for disease-specific dosing, biomarker surveillance, and mechanism-guided management rather than DLBCL-based extrapolation.
- Manufacturing speed, durability of response, and real-world scalability are emerging as core determinants of clinical impact, influencing persistence, access, and treatment feasibility beyond specialized centers.
- Current evidence remains early-phase and hypothesis-generating, limited by small cohorts, short follow-up, and single-arm designs—defining the agenda for rigorous comparative validation.
- Future progress will depend on integrating biologic precision with health-system implementation, aiming to achieve durable benefit, expanded eligibility, and equitable global access to CAR-T and next-generation cellular immunotherapies.
You Can Watch More on OncoDaily Youtube TV


