The U.S. FDA has issued a Complete Response Letter (CRL) for tabelecleucel (Ebvallo), an investigational off-the-shelf, EBV-specific allogeneic T-cell therapy. The Biologics License Application (BLA) sought approval for monotherapy use in adults and children aged ≥2 years with Epstein-Barr virus–positive post-transplant lymphoproliferative disease (PTLD) who have failed at least one prior anti-CD20–based treatment.
The CRL was not related to any issues with the drug’s safety, efficacy, or the clinical data submitted in the BLA. Rather, the FDA’s concerns stem from a routine pre-license inspection of a third-party manufacturing site. Importantly, the agency did not request additional clinical trials to support approval.
What is a Complete Response Letter (CRL)?
When the FDA reviews a new drug application, it can either approve it or issue a Complete Response Letter (CRL). A CRL indicates that the application cannot be approved in its current form, but it does not necessarily reflect concerns with the drug’s safety or efficacy.
Often, a CRL points to manufacturing, documentation, or inspection issues that must be resolved before the drug can move forward. In this case, the FDA’s concerns were limited to a third-party manufacturing facility—not the clinical data.
What Is Tabelecleucel and How Does It Work?
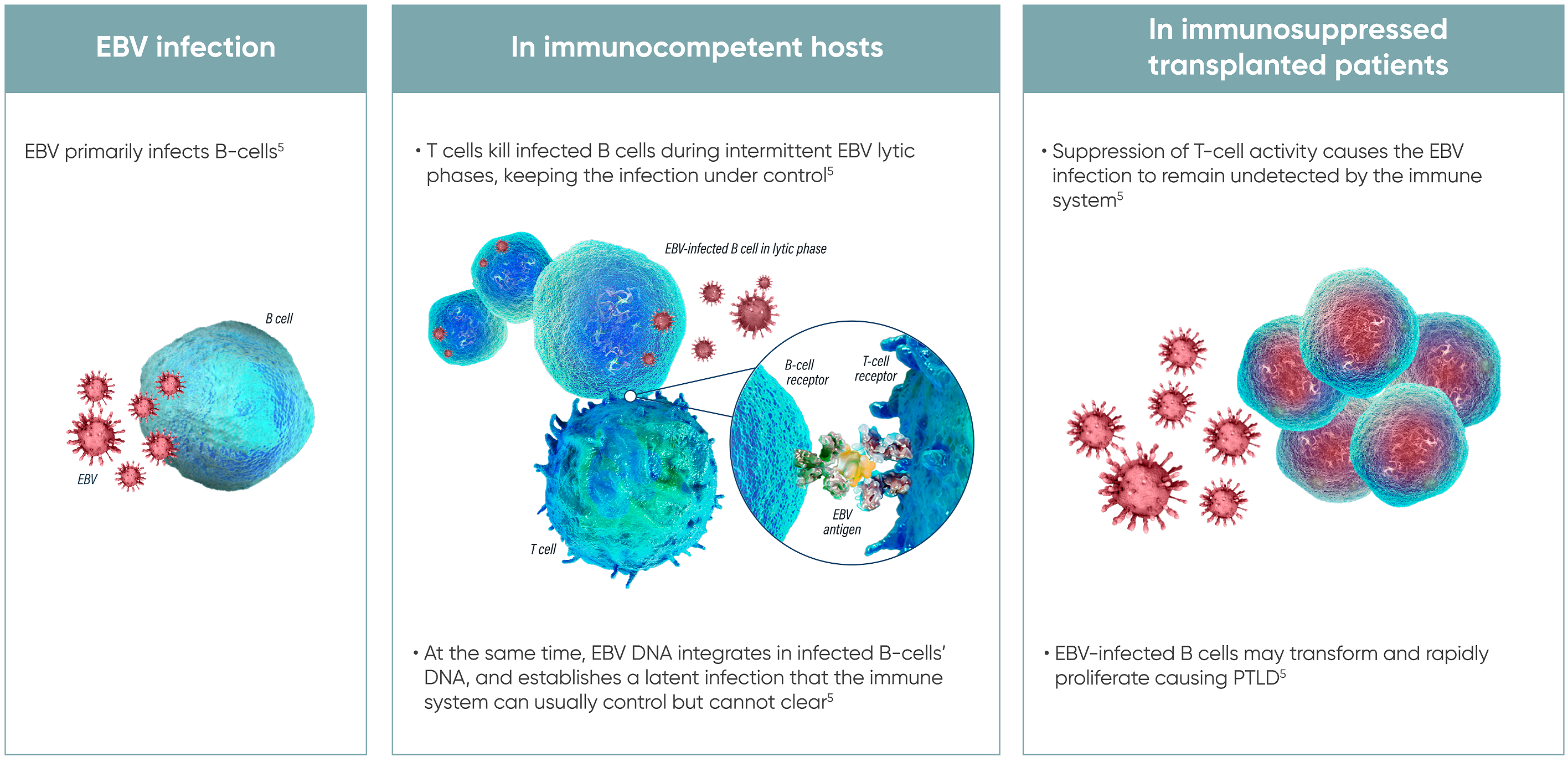
Tabelecleucel is a first-in-class, allogeneic Epstein-Barr virus (EBV)–specific T-cell immunotherapy developed for the treatment of EBV-positive post-transplant lymphoproliferative disease (PTLD). PTLD is a rare but potentially fatal complication that can occur in immunosuppressed patients following solid organ or hematopoietic stem cell transplantation.
Unlike autologous cell therapies that require manufacturing from the patient’s own cells, tabelecleucel is derived from healthy donor T cells that are pre-sensitized to EBV antigens. These cells are then partially HLA-matched to the patient and stored as an off-the-shelf therapy, enabling rapid delivery in urgent clinical situations.
Mechanism of Action
Tabelecleucel works by reconstituting EBV-specific cellular immunity in immunocompromised patients. The infused donor-derived T cells recognize and bind to EBV-infected B cells, which are the primary drivers of lymphoproliferation in PTLD. Upon engagement, these T cells initiate cytotoxic responses, directly killing the infected cells through granzyme and perforin-mediated apoptosis.
This targeted approach enables selective elimination of EBV-positive malignant cells while sparing normal tissues, offering a novel and non-chemotherapeutic strategy for patients with limited treatment options and poor prognosis.
Company Response and Resubmission Timeline
Atara Biotherapeutics and its partner, Pierre Fabre Laboratories, confirmed their intent to resolve the manufacturing compliance issues. According to Atara CEO Cokey Nguyen, the company expects to resubmit the BLA promptly once GMP compliance is achieved, with potential FDA approval within six months of resubmission.
Pierre Fabre CEO Eric Ducournau added:
” We are disappointed by the delay and are willing to work with Atara on appropriate next steps to bring tabelecleucel to US patients that suffer from this deadly rare disease with no approved therapies.”
Results from the Phase 3 ALLELE Trial
The BLA for tabelecleucel was supported by data from the Phase 3 ALLELE study (NCT03394365), a global, open-label trial in patients with EBV+ PTLD post–solid organ or hematopoietic stem cell transplantation, who were refractory to or relapsed after rituximab, with or without chemotherapy.
Participants received tabelecleucel intravenously on days 1, 8, and 15 of the first cycle. Disease assessment occurred at day 28 and every 3 months for up to 2 years.
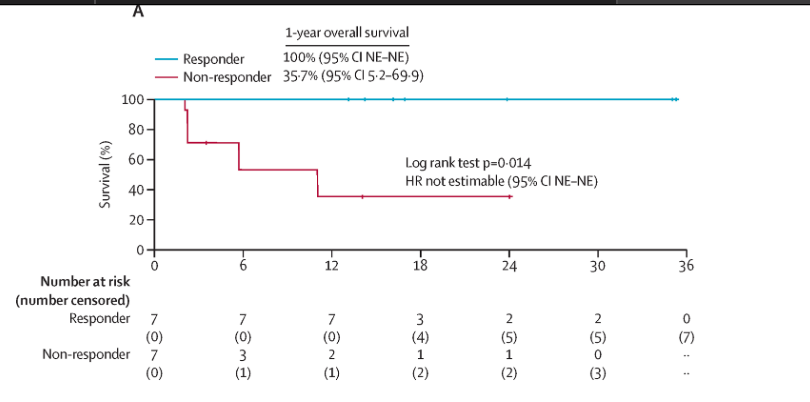
Figure 2: Kaplan–Meier curve of survival in patients with EBV-positive post-transplant lymphoproliferative
disease following HSCT or SOT who received tabelecleucel: (A) Overall survival in patients with EBV-positive post-transplant lymphoproliferative disease following HSCT.
At the 2024 ASH Annual Meeting, updated findings were presented:
-
Overall response rate (ORR): 50.7%
-
Complete response rate: 28.0%
-
Median time to response: 1.1 months
-
Median duration of response: 23 months
-
Median progression-free survival (PFS): 23.9 months
-
Median overall survival (OS): 18.4 months
-
12-month PFS and OS rates: 74.2% and 55.7%, respectively
Safety Profile
Tabelecleucel was well tolerated in the ALLELE trial. Treatment-emergent adverse events (TEAEs) were reported in 62.7% of patients, though only 8.0% were attributed to the treatment. Notably, no patients experienced cytokine release syndrome, neurotoxicity, infusion-related reactions, or immune-related complications such as graft rejection or immunogenicity. There were fatal TEAEs, but none were considered related to tabelecleucel.
Global Landscape: EMA Approval and Manufacturing in Europe
While the FDA decision delays U.S. approval, tabelecleucel is already approved in the European Union. The European Commission granted marketing authorization in December 2022, making it the first allogeneic T-cell therapy approved in Europe for EBV+ PTLD.
In addition, a second manufacturing partner, FUJIFILM Diosynth Biotechnologies, has received European Medicines Agency (EMA) approval to produce tabelecleucel for the EU market—highlighting a potential pathway for resolving supply chain challenges in the U.S.
Outlook: A First-in-Class Therapy Within Reach
Tabelecleucel represents a first-in-class EBV-specific immunotherapy for a rare, often fatal post-transplant complication with no FDA-approved treatments to date. The clinical benefit demonstrated in ALLELE—paired with a manageable safety profile and promising durability of response—positions the therapy as a potentially transformative option.
Pending resolution of manufacturing compliance issues, a successful resubmission could bring this therapy to U.S. patients as early as late 2025, offering hope in a setting where treatment options are limited.