Rituxan (rituximab) is a monoclonal antibody that targets the CD20 protein on B-cells, playing a pivotal role in oncology as a form of immunotherapy. Since its FDA approval on November 26, 1997, Rituxan has become integral in treating certain cancers, offering a targeted approach distinct from traditional chemotherapy.
How Does Rituxan Work?
Rituxan is not a chemotherapy drug. Instead, it is a chimeric monoclonal antibody that targets CD20, a surface antigen found on B cells. It works by depleting both normal and pathogenic B cells while preserving plasma cells and hematopoietic stem cells, as they do not express the CD20 antigen. Once Rituxan binds to CD20, it works in two ways by either enhancing the immune system, which can trigger processes that lead to the cancer cells’ death on its own, or directly destructing cells. The latter helps your body’s natural defenses recognize and destroy cancer cells.
What Cancers is Rituximab (Rituxan) Approved to Treat?
Rituxan is a prescription medication administered by a healthcare professional. It can be used alone or in combination with other treatments for cancerous conditions, including Non-Hodgkin’s lymphoma (NHL) and chronic lymphocytic leukemia (CLL) in adults, as well as mature B-cell non-Hodgkin’s lymphoma (NHL) and mature B-cell acute leukemia (B-AL) in children aged six months and older. Additionally, it is indicated for autoimmune disorders such as rheumatoid arthritis in adults, granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA), which are rare inflammatory conditions affecting blood vessels and tissues treated in both adults and children aged two years and older, and pemphigus vulgaris, a severe autoimmune disease causing blisters and skin breakdown, treated in adults.
Rituxan in Non-Hodgkin’s Lymphoma
Relapsed or Refractory, Low-Grade or Follicular NHL (used as a single agent). Previously Untreated Follicular NHL (used in combination with first-line chemotherapy. If patients achieve a complete or partial response, Rituxan may be continued as single-agent maintenance therapy). Non-Progressing (Including Stable Disease), Low-Grade NHL (used as a single agent after first-line CVP chemotherapy (Cyclophosphamide, Vincristine, Prednisone)). Previously Untreated Diffuse Large B-Cell Lymphoma (DLBCL) (used in combination with CHOP chemotherapy (Cyclophosphamide, Doxorubicin, Vincristine, Prednisone) or other anthracycline-based regimens).
In the AUGMENT trial, published by John P. Leonard et al. in The Lancet (2019), the combination of Rituxan and lenalidomide (Revlimid) demonstrated significant improvements in progression-free survival (PFS) compared to Rituxan alone in patients with relapsed or refractory indolent NHL. The median PFS was 39.4 months in the Rituxan plus lenalidomide group versus 14.1 months in the Rituxan plus placebo group (HR=0.46, P<0.001).
Additionally, the RELEVANCE trial, published by Gilles Salles et al. in The Lancet (2017), evaluated Rituxan in combination with chemotherapy regimens in previously untreated follicular NHL patients. The study found that Rituxan combined with chemotherapy resulted in improved PFS compared to chemotherapy alone.
These findings underscore the efficacy of Rituxan in various treatment settings for CD20-positive, B-cell NHL, highlighting its role in improving progression-free survival across different patient populations.
Rituxan for Pediatric Patients (Aged 6 Months and Older)
Rituxan is indicated for the treatment of advanced-stage, CD20-positive B-cell malignancies in children, including Diffuse Large B-Cell Lymphoma (DLBCL), Burkitt Lymphoma (BL), Burkitt-Like Lymphoma (BLL), and Mature B-Cell Acute Leukemia (B-AL). In pediatric patients, Rituxan is used in combination with chemotherapy as part of initial treatment. In pediatric patients, Rituxan is used in combination with chemotherapy as part of initial treatment.
Rituxan in Chronic Lymphocytic Leukemia
Rituxan is approved for the treatment of CLL, typically in combination with chemotherapy agents such as fludarabine and cyclophosphamide. This combination has been shown to improve progression-free survival compared to chemotherapy alone. Clinical studies have shown that this combination results in a median progression-free survival of 39.8 months, compared to 31.5 months with FC alone. The overall response rate with Rituxan plus FC is 86%, versus 73% with FC alone. In previously treated CD20-positive CLL, the combination of Rituxan and FC has demonstrated a median progression-free survival of 26.7 months, compared to 21.7 months with FC alone. The overall response rate in this setting is 54% with Rituxan plus FC, versus 45% with FC alone.
According to the CLL8 trial, published by Gatel, Krenacs, and Bohn in the Journal of Clinical Oncology (2009), this combination has been shown to improve progression-free survival compared to chemotherapy alone. The trial demonstrated that this combination results in a median progression-free survival of 39.8 months, compared to 31.5 months with FC alone.
The overall response rate with Rituxan plus FC is 86%, versus 73% with FC alone. In previously treated CD20-positive CLL, the combination of Rituxan and FC has demonstrated a median progression-free survival of 26.7 months, compared to 21.7 months with FC alone. The overall response rate in this setting is 54% with Rituxan plus FC, versus 45% with FC alone (Gatel, Krenacs, & Bohn, 2009).
Rituxan for Metastatic Cancer
While Rituxan is not specifically marketed as a treatment for metastasis, its effectiveness in advanced-stage, aggressive B-cell malignancies (such as certain types of NHL) suggests its potential in controlling metastasis by targeting cancer cells that have spread to other parts of the body.
Rituxan Combinations: Enhancing Treatment Outcomes
Rituxan (rituximab) is commonly used in combination with other treatments to enhance its therapeutic effects, particularly in cancers like Non-Hodgkin’s Lymphoma (NHL) and Chronic Lymphocytic Leukemia (CLL). Combining Rituxan with other drugs can improve efficacy, reduce the risk of resistance, and address more aggressive or advanced stages of cancer. Here, we’ll explore some of the most effective combinations and their impact on treatment outcomes.
Rituxan + Chemotherapy
CHOP (Cyclophosphamide, Doxorubicin, Vincristine, Prednisone) is a chemotherapy regimen for aggressive non-Hodgkin lymphoma (NHL), including DLBCL. It targets rapidly dividing cancer cells. Adding Rituxan enhances B-cell targeting through immune-mediated destruction, improving response rates and survival, particularly in advanced-stage or high-risk NHL. This combination is the standard first-line therapy for many aggressive NHL types.
According to a 2023 study published in The American Journal of Hematology, patients receiving R-CHOP achieved a 5-year overall survival rate of 70%, significantly higher than those treated with CHOP alone. A 2022 article in Blood Advances further highlights that the addition of Rituxan to CHOP improves progression-free survival and reduces relapse rates, reinforcing its role as the standard first-line therapy for aggressive B-cell lymphomas. These findings confirm that R-CHOP remains one of the most effective regimens for DLBCL, offering better long-term remission and survival outcomes.
CVP (Cyclophosphamide, Vincristine, Prednisone) is used for indolent, low-grade B-cell NHL, such as follicular lymphoma, by inhibiting cancer cell growth. Adding Rituxan improves outcomes by targeting CD20 on B-cells, enhancing progression-free and overall survival in relapsed or refractory follicular lymphoma and other B-cell malignancies.
A pivotal study published in Blood demonstrated that patients receiving R-CVP had an overall response rate of 81%, with a complete response rate of 41%, compared to 57% and 10%, respectively, in the CVP-only group. This study also reported a median time to treatment failure of 27 months for the R-CVP group versus 7 months for the CVP group, highlighting the substantial benefit of adding rituximab to the regimen.
Further research has explored the benefits of maintenance therapy with rituximab following initial treatment. A meta-analysis published in the Journal of the National Cancer Institute indicated that maintenance rituximab therapy improved overall survival in patients with relapsed or refractory follicular lymphoma. However, this benefit was not observed in previously untreated patients. The analysis also noted an increased rate of infection-related adverse events associated with maintenance therapy, suggesting that the decision to implement maintenance rituximab should be individualized based on patient history and risk factors.
Recent advancements in treatment combinations have also been investigated. A study highlighted in Blood Advances examined the prolonged use of rituximab maintenance in follicular lymphoma patients, discussing long-term outcomes and considerations for extended therapy.
Rituxan + Immunotherapy
FAQ
Can Rituxan Be Used in Autoimmune Diseases?
Yes, Rituxan (rituximab) treats autoimmune diseases like rheumatoid arthritis, granulomatosis with polyangiitis, and microscopic polyangiitis. It works by depleting B-cells to reduce inflammation and immune attacks. It's usually prescribed when other treatments fail.
How Long Does It Take for Rituxan to Show Results?
The time for Rituxan to show results varies by condition. In cancers like NHL and CLL, improvements may be seen within a few weeks, but takes months for full effect. In autoimmune diseases, relief may begin within 4-6 weeks, with full benefits requiring multiple cycles. Regular follow-ups will help assess progress
Can Rituxan Cause Long-Term Immune Suppression?
Yes, Rituxan can lead to long-term immune suppression, as it targets B-cells. This may increase the risk of infections, especially during and after treatment. Regular check-ups and preventive care are important to manage this potential side effect.
Are There Dietary Restrictions While Taking Rituxan?
No strict restrictions, but a balanced diet is key. Avoid alcohol, unpasteurized foods, and high-sodium intake. Stay hydrated and focus on nutrient-rich foods like fruits, vegetables, whole grains, and lean proteins to support your immune system during treatment. Always consult your doctor for personalized advice.
What Are the Common Side Effects of Rituxan?
Common side effects include infusion reactions (fever, chills, nausea), fatigue, infections, low blood counts, and rash. Serious effects, like severe infections or heart complications, require immediate medical attention
Does Rituxan Affect COVID-19 or Other Vaccines?
Yes, Rituxan can weaken vaccine responses due to B-cell depletion. It's best to complete necessary vaccinations before starting treatment and consult your doctor regarding COVID-19 boosters.
Can Rituxan Cause Hair Loss?
No, Rituxan itself does not typically cause hair loss, unlike chemotherapy. However, hair thinning may occur due to other medications used alongside Rituxan.
How Long Do Rituxan’s Effects Last?
B-cell depletion can last 6 to 12 months post-treatment. Some patients remain in remission for years, while others may need maintenance therapy.
Can You Take Rituxan While Pregnant or Breastfeeding?
Rituxan is not recommended during pregnancy as it may affect the baby’s immune system. If needed, birth control is advised during treatment and for 12 months after the last dose. Breastfeeding is also not recommended.
Does Rituxan Interact with Other Medications?
Yes, Rituxan may interact with immunosuppressants, vaccines, and certain blood pressure medications. Always inform your doctor about all medications you are taking.
What are Rituximab Administration Guidelines?
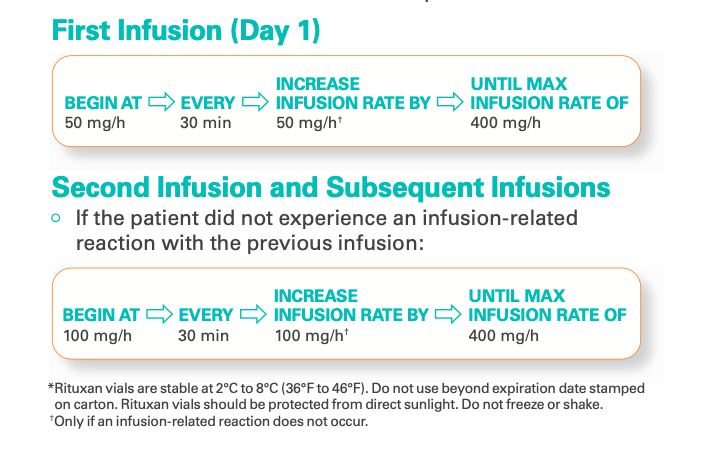
Rituximab is administered as an intravenous (IV) infusion, which starts at 50 mg/hour and can be gradually increased to a maximum of 400 mg/hour if tolerated. Rituximab should never be given as an IV push or bolus.
What is the Dosage of Rituximab for CLL?
For chronic lymphocytic leukemia (CLL), Rituximab is given at 375 mg/m² IV on day 1 of the first cycle, followed by 500 mg/m² IV on day 1 of each subsequent cycle, in combination with chemotherapy. The treatment is typically repeated every 28 days for up to six cycles. Always discuss with your treating physician.
What is the Dosage of Rituximab in CHOP?
In the CHOP regimen (cyclophosphamide, doxorubicin, vincristine, and prednisone) for non-Hodgkin’s lymphoma (NHL), Rituximab is administered at 375 mg/m² IV per cycle, typically on day 1, before chemotherapy. The treatment is usually repeated every 21 days for six to eight cycles, depending on the disease stage and response.